Principles, analytical framework and resources on farms for integrated health management in monogastric animals (Full text available in English)
Header
Promoting the building up of animals’ health throughout their lives makes it possible to continue to reduce the use of antibiotics and antiparasitic agents in animal production and thus limit the development of resistance to these compounds. We discuss here the practices that are available in animal production that help meet this objective. However, development of farming systems that meet societal demands (respect for animal welfare, short and local distribution channels, access to the outdoors) poses new challenges for integrated animal health management.
Introduction
Controlling animal health (Box 1) is essential in animal production and addresses a threefold challenge: optimising the production cycle and reducing losses (economic challenge), contributing to the well-being of animals by taking care of them (ethical challenge) and limiting the emergence of zoonoses (public health challenge). Since their discovery in the 1930s, antibiotics and antiparasitic agents, which respectively control infectious diseases of bacterial origin and parasites, have been essential elements of animal health management. In animal production, they are used to treat an infected animal (individual curative treatment) or a group when some of a batch is ill (metaphylaxis; Lhermie et al., 2015). In October 2018, the European Parliament ruled against the preventive use of antibiotics, i.e. before the onset of disease, by treating all animals in a batch that has a high probability of a disease occurring. Indeed, their massive use in animal production (Anses, 2020) has contributed to the emergence of resistance that reduces their effectiveness in animals and can be transmitted to humans, either through human-animal proximity or via the food chain. This is why the fight against antibiotic resistance has become a global public health challenge that has been translated into two national action plans (EcoAntibio: 2012-2017 and 2017-2021; https://agriculture.gouv.fr/le-plan-ecoantibio-2-2017-2021)
Box 1. Animal health, disease and welfare.
Although there are multiple definitions of “animal health” (Gunnarsson, 2006), there is no consensus for that of farm animals. Traditionally, farm animal health was defined in opposition to disease, which was understood as a deterioration in health, and was frequently assessed using “production traits” (growth, reproduction, etc.; Villemin, 1981). However, as early as 1946, the World Health Organization defined health more broadly as a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.
From a physical viewpoint, disease occurs as a result of exceeding the adaptive capacity to cope with i) the action of pathogens (bacteria, viruses, parasites, fungi), ii) exposure to toxic substances (mycotoxins, xenobiotics) and iii) living conditions that are not adapted to the needs of the animal (climate, atmosphere, stress, impoverished living environment, etc.). In addition to infectious diseases, "production diseases" are multifactorial diseases, which may also be infectious, that influence the health and well-being of animals and decrease their productivity and that of the farms. They have also been defined as persistent and to increase with the intensification of animal production (Le Floc'h et al., 2021).
Thus, animal health is not only the absence of disease, but a form of resilience expressed as an animal's ability to maintain a physiological and psycho-emotional balance in an environment, including microbial (living with its pathogens), that is temporary and sometimes challenging (Döring et al., 2015). The psychosocial health of animals requires further research, with consideration of the cognitive abilities and needs of animals, including social needs. However, a connection between stress and animal physiology has been demonstrated (Fraser et al., 2013).
Anses (2018)1 defines the welfare of an animal as the positive mental and physical state related to the satisfaction of its physiological and behavioural needs and expectations. This state varies according to the animal's perception of the situation. Health and welfare are thus two concepts that overlap but are not the same.
Ultimately, health is both a state of homeostasis that allows for the optimal performance of biological functions and a process of maintaining or restoring this homeostasis in response to changes in the living environment. It is defined and assessed at the individual or group level.
URL: https://www.anses.fr/fr/glossaire/1535. Accessed on 8 June 2021.
Monogastric animal species are particularly concerned by these issues. At present, these species are reared mainly in highly organised systems with high animal densities and artificial living environments, and their farms use large amounts of antibiotics (Anses, 2020). In the same issue, Paul et al. (2021) review the evolution of antibiotic use in the monogastric animal sector and present the approaches developed to reduce its use. The objective of this article is to define the principles, develop an analytical framework and identify the practices available at the animal and farming system levels for integrated health management of monogastric animals, mainly pigs, rabbits and poultry. We illustrate this by showing how various practices can be combined to meet this objective and conclude by discussing limitations of current conventional systems and the challenges they must address in pursuit of demedicalisation.
1. Definition and principles of integrated animal health management
1.1. Definition and purpose
Integrated animal health management is a relatively recent concept. As described by Dumont et al. (2013), it is defined as all the knowledge and practices that humans use in a coordinated manner to build up, maintain or restore the health of an animal or herd within a farming system (farming system: Box 2). While the farmer is a central element, this coordination can also result from a work group involved directly or indirectly on the farm (veterinarian, advisor, employee, etc.; Manoli et al., 2021; Gotti et al., 2021a). Integrated animal health management refers to a comprehensive approach to health, i.e. a multifactorial vision, derived from work on ecopathology (Ganiere et al., 1991). It is based on regular monitoring of the farm and its animals: assessing farm management, analysing risk practices and implementing risk management plans and adjustments. The aim is to maintain and/or restore the herd's health balance.
The aim of integrated animal health management is i) to promote the building up of animal health so that animals have a harmonious life trajectory and are in a state of well-being and ii) to prevent the occurrence of diseases to reduce the use of medicines (antimicrobials, anthelmintics, etc.). Improving animal welfare contributes to this goal (Box 1). From the farmers' viewpoint, the objective is to optimise the production cycle and reduce losses (economic and animal life) in animal production due to diseases. More generally, it is a question of preserving human health (zoonoses and antibiotic resistance) and the health of ecosystems (“One Health” concept; Hickman et al., 2021).
Integrated animal health management involves an initial phase of farming system design followed by continuous and iterative phases of assessing health (individual and groups of animals) and adapting the functioning of the farming system over time to achieve the goal of healthy animals (health balance).
The design concerns the basic choices that are based on the resources available and the structural constraints of the farming system. The size and organisation of the buildings, the layout of the animals’ living environment, the genetic type of the animals and the choice of mechanisation and/or automation have long-term consequences. These choices shape the work on the farm and the functioning of the biotechnical system and must be consistent with each other and adapted to the objectives of the system. Animal health assessment is a systemic approach (https://dicoagroecologie.fr/en/encyclopedie/ecopathology/) based on observing the animal or group of animals in their environment. It aims to identify physical, physiological or behavioural disorders; their causes; and risky practices in order to avoid focusing on treating the effects of disease (identifying the cause rather than treating symptoms). It can extend to diagnosing diseases based on clinical examination and observation of animal or herd behaviour or of the living environment. The adaptation phase consists of revisiting production practices (feeding strategies, reproduction rates, prophylaxis, culling criteria) to achieve short- or medium-term goals or even to modify certain elements of system design (choice of genetics, housing type, etc.), if necessary. This phase is based on the farmer’s production and business objectives, which are related to the structural, economic and technical conditions of the farm (Box 2).
Box 2. The concept of an animal farming system.
An animal production system is a set of dynamically interacting elements, organised by humans according to their objectives, to produce (milk, meat, hides and skins, labour, manure, etc.) and reproduce a group of domestic animals by developing and renewing different resources (Dedieu et al., 2008). It consists of a decision-making system (the farmer or a work group) that manages the biotechnical system (the animal system(s), which may be associated with a forage or field-crop system). Most current pig, rabbit and poultry farming systems are described as “confined” because the animals' feed is not produced on site. In this situation, the biotechnical system managed by the farmer is restricted to animal production systems, which are spatially restricted to animal buildings if the animals have little or no access to the outdoors.
An animal production unit is a group of animals in their rearing environment that provide a product of the same type and are managed in the same way (Menjon and d'Orgeval, 1983). For example, in farrow-to-finish pig or rabbit farming, the breeding female system is separate from the growing animal system. In poultry farming, the laying hen system is separate from the broiler breeding system (reproduction), and the hatching system (egg incubation and chick hatching) is separate from the breeding system.
The farmer (or the work group) aligns his/her (or its) objectives (economic, environmental, social, production services, other services) with the management of the breeding system. This management is based on information from the animal production units (e.g. production performance, animal health). Farm animal practices are the indicators of the farmer's decisions. Combining the biotechnical system and the decision-making system is dynamic, with time steps that depend on the risks that occur in the system's environment (Dedieu and Ingrand, 2010): choices can be tactical, at an annual or production-cycle scale, or more strategic, at a longer time scale, which results in greater reconfiguration of the system.
Farmers' actions are influenced by many factors. Their activities are thus governed by economic constraints and environmental regulations, but also by social norms of the professional world in which they evolve: their farmer peers and other actors in the production chain (technicians, veterinarians, salespeople) who define “good practices”, for example for animal health management or how to improve the yields of their farms (Darré et al., 2004; Compagnone). Factors such as risk aversion also have a strong influence on health-management practices and antibiotic use (Ducrot et al., 2018; Paul et al., 2021).
In practice, integrated health management is based on combined use of three complementary principles (P): preventing the occurrence of diseases (prophylaxis and biosecurity) (Prevent, P1); if contact with pests cannot be avoided, selecting resistant animals or developing their adaptive capacity so they become tolerant to pests (Resist or tolerate, P2); and, if the disease does occur, treating the animals in a well-reasoned way (Treat, P3). These three principles are developed below.
1.2. Prevention (Principle 1)
Prevention consists of avoiding risky situations, i.e. those that are likely to exceed the animals’ adaptive capacity (contact with pathogens, discomfort, aggression, etc.). Providing a suitable living environment and implementing practices that meet the physiological and behavioural needs and expectations of the animals are essential to prevent disease. In practical terms, various practices are available to achieve this objective (section 3.1).
Prophylaxis refers to the methods used to monitor the health of an individual or population and prevent the onset, spread or aggravation of diseases. It includes practices for monitoring individuals and herds through analysis and the use of health-monitoring tools. For transmissible infectious diseases, disease control is also based on applying the principles of i) external biosecurity, which aims to prevent and/or decrease the introduction of new microbial, viral or parasitic strains onto the farm. This is achieved by checking the health of animals when they enter the farm and implementing physical barriers or traps that prevent the presence of vectors (insects, rodents, etc.), as well as ii) internal biosecurity, which consists of methods to reduce the spread of pathogens on the farm (Corrégé and Hémonic, 2018). Isolating and/or eliminating infected and potentially contagious animals (whether they are sick or not) requires organising animal movement to reduce the spread of pathogens on the farm, as well as cleaning and disinfection protocols for farm buildings and equipment to reduce the presence of pathogens. However, most microorganisms in the environment or hosted by animals (digestive and skin microbiota, etc.) are not pathogenic. In contrast, symbiotic or commensal microorganisms can help reduce the development of pathogens in animals (Ducarmon et al., 2019). Orientating the environmental microbiota is a strategy that can reduce the risk of disease. Besides biotic risks, abiotic risks (air quality, dust, temperature, etc.) must also be controlled to prevent health problems. However, the problems and methods available depend strongly on whether the animals are confined or have access to the outdoors. Thermal comfort is easier to control in confined housing, but this comes at a significant cost. Respiratory problems related to ventilation and air quality occur less frequently on farms with access to the outdoors.
1.3. Resist and/or tolerate (Principle 2)
Schneider and Ayres (2008) defined resistance as the ability of an organism to limit the pathogen load, and tolerance as the ability of an organism to limit the health effects of a pathogen. These definitions were established for infections but can also be applied to other conditions such as thermal stress (Berry and López-Martínez, 2020). Råberg et al. (2007) suggest that resistance involves a process of antagonistic coevolution between host and pathogen. Tolerance is more likely to result from mechanisms that regulate the damage caused to a host by its immune system responding to pathogens. Robustness is an animal's ability to maintain its physiological functions and a state of health considered acceptable in a wide variety of environments (availability of resources, climate conditions, etc.; Blanc et al., 2013).
Selecting robust genotypes (Friggens et al., 2017) or using lines selected for disease resistance (Ducos et al., 2021) are two relevant strategies in integrated animal health management. Genetic resistance can be specific (salmonella in chicken; Tran et al., 2012) or broader (Gunia et al., 2018), but no species is resistant to all diseases. Stimulating the adaptive capacity of animals in response to biological agents or environmental variations is thus an essential complementary strategy to maintain animal health. When available, vaccination can protect animals against specific pathogens (myxomatosis or viral haemorrhagic disease (VHD) in rabbits; Marek's disease, Gumboro disease or infectious bronchitis in poultry; circovirus type 2 in pigs). Unlike innate immunity, the maturation of animals' adaptive immune response can also be stimulated by exposing them to a rich microbial environment from an early age (Round and Mazmanian, 2009). This contributes to the diversity of digestive microbiota and development of the immune system associated with the gut, especially diversification of the antibody repertoire (Lanning et al., 2000). This practice interacts strongly with animal diet, especially the intake of dietary fibre and prebiotics, which are substrates that contribute to the development of a digestive microbiota beneficial to its host. The balance of amino acids and macro- and micronutrients in the diet is also essential to maintain the cellular redox balance of animals and to avoid generating oxidative stress and chronic inflammation, which can lead to health problems (Durand et al., 2021). Ultimately, resistance and tolerance should be controlled together to optimise the building up of animal health.
1.4. Treat sensibly (Principle 3)
Applying the above principles is not always sufficient to prevent the occurrence of disease. Therefore, caring for animals by treating symptoms and combatting infectious agents is necessary. For non-infectious diseases (metabolic diseases, behavioural disorders, xenobiotic toxicity), animal care requires establishing rearing conditions that are conducive to recovery (suitable diet, isolation or socialisation, environmental conditions, adapting the living environment, etc.) and managing injuries. To treat infectious diseases, some farms, especially those in “organic” systems, prioritise alternative treatments over veterinary medicines (Hellec et al., 2021), such as phytotherapy (Blanco-Penedo et al., 2018) or aromatherapy (Zhai et al., 2018) before applying allopathic treatments. These strategies aim to avoid early exposure to antibiotics that alter the digestive microbiota, enteric nervous system and long-term metabolism (Foong et al., 2020). Others recommend early allopathic treatments to rapidly stop a disease from worsening and spreading within a group. When allopathic treatments are deemed essential, it is important to use specific medicines (antibiograms or coprocultures should be performed before treatment) rather than broad-spectrum medicines and to follow the prescribed protocol (dose, target population, duration of treatment and withdrawal period) to minimise the amounts used. A veterinarian is the only person on a farm who can prescribe medicines.
For monogastric animal production, health management is performed more often at the group level than at the individual level. This is due to the collective rearing methods (in hutches), the sometimes-moderate economic value of an individual and the large size of the group to be managed. This is why, for these species, it is essential to follow the first two principles of integrated health management to reduce the amount of medicines used.
In the remainder of this article, we focus on practices that can be used at the farming system level for integrated animal health management. Other practices are applicable at a larger level, such as the region (e.g. movement or exchange of animals or equipment within an area, monitoring animal health within a region; Madderm et al., 2012) or the production chain (e.g. traceability or consistent practices between links in the chain, differing roles of various veterinary advisors), but are not discussed. In addition, Paul et al. (2021) analysed concrete actions implemented in the field to help reduce antibiotic use.
2. A conceptual framework for representing animal health
2.1. The components of animal health
To be able to consider the integrated management of monogastric animal health beyond the characteristics of each species, the RIMEL consortium (Fortun-Lamothe et al., 2017) developed a conceptual framework of the components of animal health defined above (Box 1). Its objective is to identify the structural elements that help build up the health of farm animals to integrate it into the knowledge required for integrated management. This framework includes physical and psychosocial dimensions, which are subdivided into 11 components (Figure 1). The physical dimension of health includes physical barriers that interface with the external environment (integument, mucous membranes and microbiota) and the defence system, which consists of the immune, nervous and endocrine systems. The psychosocial dimension includes social connections between individuals (mother-offspring, between congeners), the expression of innate behaviours and respect for the circadian rhythm. These components interact with each other and with the organs involved in major biological functions (section 2.2).
Figure 1. Conceptual framework of the components of animal health.
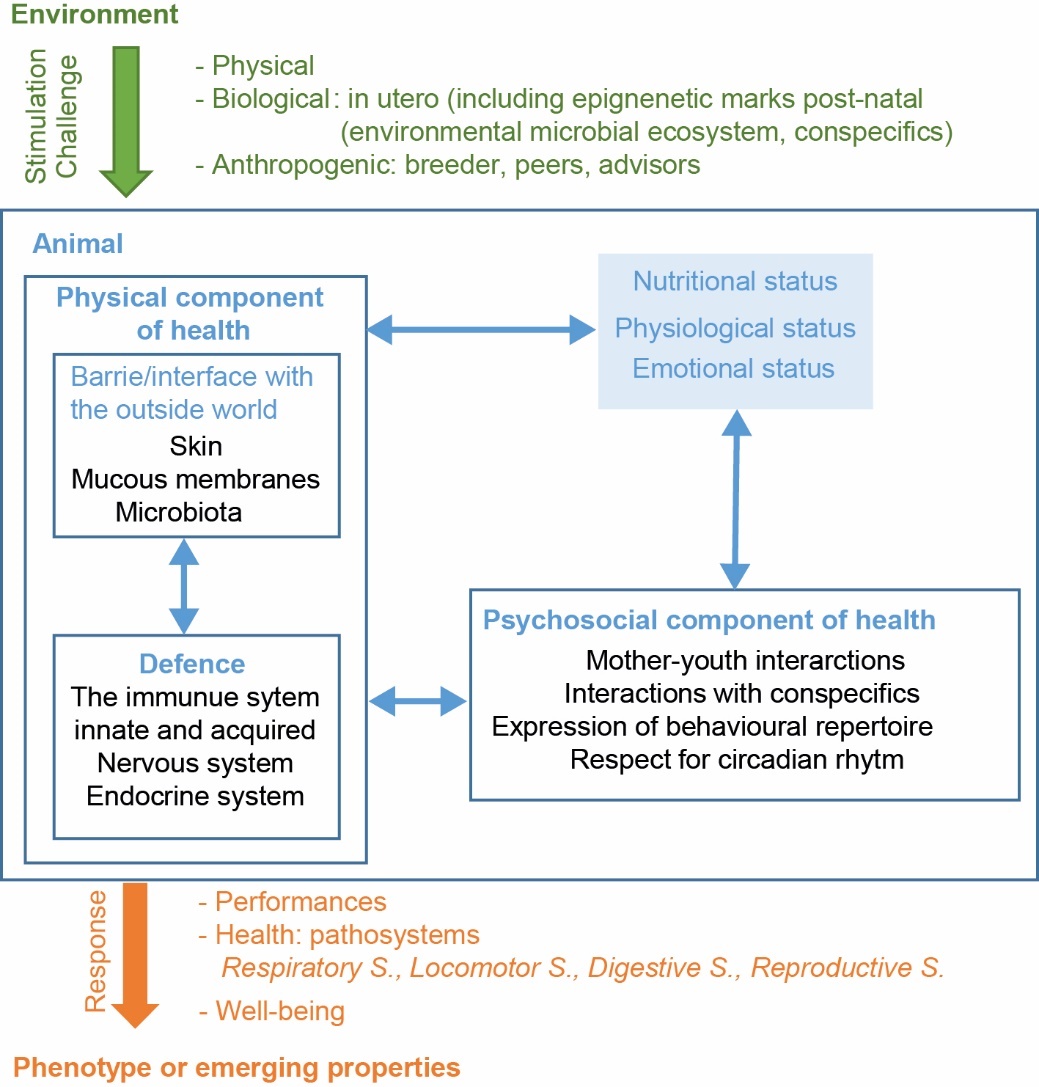
Species-specific periods have been identified as critical for health (e.g. perinatal period, weaning) as they correspond to key periods of exposure of animals to high risks at a time when certain components may not have developed completely. For example, most animal species are born with immature immune systems and must rely on maternal immunity (via the yolk, placenta, or milk secretions) in the early stages of life to cope with exposure to multiple biotic and abiotic stressors in the surrounding environment (Brambell, 1970). Similarly, the adaptive immune system of mammals generally does not mature until after weaning (Weström et al., 2020). This event, which involves a change in diet, separation from the mother and sometimes a change in the living environment, represents a risk. The adaptive capacity of young animals can thus be exceeded easily. Even outside these risk periods, health components are influenced by factors intrinsic to the animal (genetics; nutritional, physiological and emotional statuses) which are influenced by the environment (biotic, abiotic and anthropogenic components). These extrinsic factors can positively or negatively influence the building up of health, sometimes in different ways depending on the developmental dynamics of the health components (section 2.3). Integrated health management aims to influence these factors according to Principles 1 and 2 described above and illustrated in section 3. Farmers are not always able to influence certain factors (e.g. climate risks in free-range farming). In these situations, integrated health management implies precaution (e.g. providing shelter) or anticipation as a part of integrated health management.
2.2. Interactions between components
The various components of animal health constantly interact. For example, the immune system develops in close interaction with the body's mucous membranes and their associated microbiota. Likewise, colonisation of the gut by the digestive microbiota is essential for development of digestive capacity and maturation of the immune system associated with the gut mucosa (Weström et al., 2020). Similarly, there are strong interactions between digestive microbiota and the central and enteric nervous systems (Foong et al., 2020) and the endocrine system. Thus, metabolites from the microbiota can influence the neuroendocrine system through different pathways (Rabot, 2015) and, conversely, the stimulation of the corticotropic axis observed at weaning helps shape the gut microbiome and modifies the metabolome of piglets (Jiang et al., 2020). A recent study suggests that pecking behaviour in hens is influenced by an interaction between gut microbiota and the central serotonin system, as well as by modulation of the immune system via the cholinergic system (Falker-Gieske et al., 2020). Furthermore, the interaction between the psychosocial component and the endocrine system can be used by pathogens that have the ability to produce or use neuromodulators and influence the behaviour of infected animals (Lyte, 2013). In pigs, transport and social stress are associated with the reactivation and spread of Salmonella Typhimurium infection via norepinephrine, a catecholamine involved in the stress response, which is thought to activate growth and expression of virulence factors in Salmonella (Pullinger et al., 2010). Finally, the interaction between the circadian rhythm and the microbiota may result in a difference in the implantation of gut microbiota in chicks depending on the photoperiod of the buildings in which they are reared (Hieke et al., 2019).
2.3. A dynamic vision of the building up of health
The DoHAD (Developmental origin of Health And Diseases) concept supports the idea that health is built up in a dynamic process that starts at gametogenesis, including the entire period of foetal and postnatal development, and ends around sexual maturity (Suzuki, 2018). Thus, the parents' living environment influences the health of their offspring through gamete development and the maternal environment during gestation and lactation (Nilsson et al., 2018). For example, dietary restriction in hens influences the immunity of their offspring (Bowling et al., 2018). Similarly, in conventional rabbit farming, rabbits are simultaneously pregnant and lactating. The superposition of lactation and gestation impacts foetal growth (Fortun-Lamothe et al., 1999) and the development of unborn rabbits (Fortun-Lamothe et al., 2000).
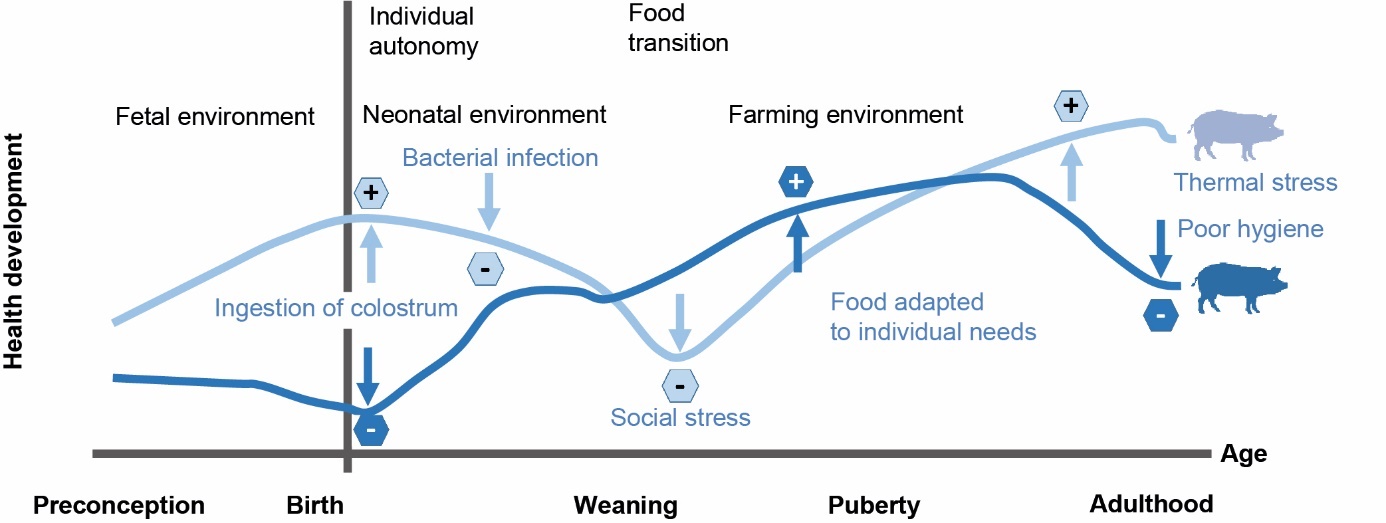
Beyond the parental imprint, exposure to favourable or unfavourable situations during development varies among individuals and generates a unique trajectory of strengthening or weakening of health (Figure 2; Halfon et al., 2014). Hertzman and Power (2011) suggested three ways to model effects of the life trajectory on health, two of which are relevant to farm animals. The latent model highlights the relationships between exposure at a given point in life and health years later. In animal production, this model applies to the early programming of phenotypes. For example, the incubation temperature of eggs alters the heat tolerance of chickens (Loyau et al., 2015). In addition, the hours after hatching/birth and the start-up/lactation phase are critical for subsequent health (Guilloteau et al., 2019; Foury et al., 2020). The cumulative model identifies combined effects on health due to multiple exposures throughout the life trajectory. This is the case for pigs, which develop a higher vaccine response when their immunity has been stimulated by hygienic housing conditions (Chatelet et al., 2018). Ultimately, the available knowledge shows that building up an animal's health is an active process involving distinct adaptation mechanisms at different stages of development that coordinate the interactions between all components of an animal's health.
Figure 2. Individual trajectories for building up an animal's health when influenced by events that occur at different stages of the animal's life (adapted from Halfon et al., 2014).
3. Practices available at the farming system level
3.1. Practices that can be used in animal production
The practices are divided into six dimensions. The first five dimensions are biotechnical and concern 1-the living environment (Table 1): its structure and organisation and the implementation of hygiene and biosecurity rules; 2-management and control of reproduction (Table 2): management and control; 3-herd management (Table 3): its structure and control; 4-practices related to the animals themselves (Table 4): the choice of genetic type, management of physical integrity and implementation of prophylaxis; and 5-feeding (Table 5): feed composition, feeding method, method and schedule of distribution and individualisation (precision feeding). Tables 1 to 5 list concrete examples of practices that can be used in the field to improve the health of pig, rabbit and poultry farming systems, and mention the integrated health management principle to which they relate (P1: prevent or P2: resist/tolerate). The sixth dimension is related to the decision-making system (Table 6): acquiring the knowledge required to implement integrated health management (biology, disease, biosafety rules, use of antibiotics, etc.), using skills related to animal health, acquiring information on health on the farm, organising equipment and work, and integrating into networks.
Table 1. Examples of environmental practices for integrated animal health management of monogastric animals at the farming system level (P: pigs, R: rabbits, P: poultry).
Dimension |
Examples of positive health effects |
Related principles a |
|---|---|---|
Structure and organisation |
Provide housing with sufficient surface area and height to allow the animals to move around and to encourage the expression of species-specific behaviours to avoid mental suffering and muscular problems (P, L, V) |
P1 |
Enrich the living environment of the animals (materials to gnaw, dig |
P1 |
|
Use suitable floors to decrease injuries and pododermatitis (P, L) |
P1 |
|
Provide sufficient clean bedding to decrease pododermatitis and digestive or respiratory problems (V), or for sows (P) and nests (L) |
P1 |
|
Use movable buildings on runs to decrease the concentration of pathogens and parasites (P, L, V) |
P1 |
|
Provide access to an outdoor grass run to meet the need for grazing (L, V) |
P1 |
|
Provide access to natural light to improve vitamin D production (P, L, V) |
P1 |
|
Use suitable materials to avoid injuries (P, L, V) |
P1 |
|
Limitations identified: Monitoring and handling animals is more complex when the housing is larger and more complex. Cleaning may be more difficult and work ergonomics may be degraded if the housing is more complex. Health risks increase, and predation is possible on the outdoor run. |
||
Biosafety, hygiene and the environment |
Optimise ventilation, humidity and rearing temperature to decrease respiratory, digestive or skin disorders (P, L, V) |
P1 and P2 |
Regular cleaning and/or sanitation to eliminate or decrease pathogen pressure and risk of infectious disease (P, L, V) |
P1 and P2 |
|
Control pests to avoid transmission of pathogens (P, L, V) |
P1 |
|
Provide a foot bath at the entrance to the farm, buildings or rooms to avoid introducing pathogens via footwear or vehicles (P, L, V) |
P1 |
|
Use specific footwear and clothing for each building or room to avoid introducing pathogens (P, L, V) |
P1 |
|
Provide washbasins and showers at the entrance to the site to avoid introducing pathogens (P, L, V) |
P1 |
|
Regularly purge and disinfect water pipes to decrease contamination (P, L, V) |
P1 and P2 |
|
Limitations identified: Loss of the barrier effect and environmental impacts |
a Principles of integrated animal health management (section 1). P1: avoid risk situations and contact with pests; P2: resist or tolerate pests
Dimension |
Examples |
Related principles a |
|---|---|---|
Reproductive management |
Wean youngsters at a late age (> 30 days) to decrease post-weaning digestive disorders (P, L) |
P1 |
Use a semi-intensive (artificial insemination 11 d after kindling) or extensive breeding schedule to reduce female mortality caused by metabolic exhaustion (L) |
P1 |
|
Avoid breeding females at too early an age to reduce metabolic disorders and disruptions of homeostasis (P, L, V) |
P1 |
|
Breeding control |
Use artificial insemination rather than natural mating to decrease the transmission of sexually transmitted diseases (P, L, V) |
P1 |
Perform controlled suckling to reduce injuries to young rabbits and detect suckling defects (L) |
P1 |
|
Practice self-renewal of breeding stock or have a herd of grandparent animals to avoid introducing external pathogens when renewing breeding stock (L) |
P1 |
|
Limitations identified: All animals in the flock or herd may be susceptible at the same time (transmission of pathogens) |
a Principles of integrated animal health management (section 1). P1: avoid risk situations and contact with pests; P2: resist or tolerate pests
Table 2. Examples of reproductive management practices for integrated animal health management of monogastric animals at the farming system level (P: pigs, R: rabbits, V: poultry).
Table 3. Examples of herd management practices for managing integrated monogastric animal health at the farming system level (P: pigs, R: rabbits, V: poultry).
Dimension |
Examples |
Related principles a |
|---|---|---|
Herd formation |
Manage (observe, avoid, advance, delay, etc.) the entry and exit of animals in and out of the flock, herd or farm to reduce the presence of animals at risk for a health or behavioural disorder (P, L, V) |
P1 |
Avoid moving breeding animals between batches to decrease the transmission of pathogens (L, V) |
P1 |
|
Manage animals in batches to use more refined feeding strategies (P, L, V) |
P1 |
|
Manage animals in batches to perform cleaning and disinfection after each batch (P, L ? V) |
P1 and P2 |
|
Limitations identified: Single-batch management can impose unproductive periods that reduce individual productivity. In batch farming, all animals have the same physiological stage and are thus susceptible at the same time, which can promote the spread of diseases. |
||
Control and monitor animals in the herd |
Homogenise the size of litters at birth via adoption between litters to reduce metabolic strain on the females and provide sufficient suckling for the young (L) |
P1 |
Decrease animal density to reduce aggression and injury and promote movement (alternative systems, P, L, V) |
P1 |
|
Manage incubation and start-up/hatching in buildings (V) |
P1 |
a Principles of integrated animal health management (section 1). P1: avoid risk situations and contact with pests; P2: resist or tolerate pests
Table 4. Examples of animal-specific practices for integrated animal health management of monogastric animals at the farming system level (P: pigs, R: rabbits, P: poultry).
Dimension |
Examples |
Related principles a |
|---|---|---|
Breed or genetic type |
Select for disease resistance (P, L, V) |
P2 |
Choose animals from more robust lines (P, L, V) |
P2 |
|
Use intermediate to slow-growing lines to reduce musculoskeletal disorders (V) |
P1 and P2 |
|
Physical integrity |
Do not trim beaks, to reduce pain (V) |
P1 |
Do not dock tails, to reduce pain and infection (P) |
P1 |
|
Castrate animals to decrease mounting and aggression between animals (P) |
P1 |
|
Remarks or limitations identified: Anaesthesia has been mandatory since 1 Jan 2022. If beak trimming and tail docking are not performed, pecking and tail biting should be managed, especially if densities are high. |
||
Prophylaxis: vaccines, phytotherapy, aromatherapy |
Vaccinate against myxomatosis and VHD (L); Marek's disease, Gumboro disease, infectious bronchitis and coccidiosis (V); and E. coli (P) |
P1 and P2 |
Use essential oils for digestive or respiratory disorders (P, L), to manage stress, stimulate the immune system and support anti-infectious properties (V) |
P1 |
|
Use herbal extracts to support liver function around parturition (P, L), support immunity and regulate the redox balance and inflammation (V) |
P1 |
|
Remarks or limitations identified: Contamination should be monitored (live attenuated vaccines) |
a Principles of integrated animal health management (section 1). P1: avoid risk situations and contact with pests; P2: resist or tolerate pests
Table 5. Examples of feed-related practices for integrated animal health management of monogastric animals at the farming system level (P: pigs, R: rabbits, P: poultry).
Dimension |
Examples |
Related principles a |
|---|---|---|
Feed composition |
Use feeds formulated to meet the nutritional needs of the animals at each physiological stage (energy, amino acids, essential fatty acids, fibre, vitamins, minerals) |
P1 |
Use highly digestible low-protein feeds with balanced amino acids to reduce post-weaning digestive disorders (P) |
P1 |
|
Incorporate phenol-rich raw materials (sainfoin) to control/prevent intestinal parasitism (L) |
P1 and P2 |
|
Supplement feed with pre- and probiotics b, essential oilsb or organic acidsb to reduce non-specific infectious disorders (V, L, P) |
P1 and P2 |
|
Supplement feed with micronutrients (vitamins, minerals) and antioxidants to reduce metabolic disorders in breeding and growing animals (L, V, P) |
P1 |
|
Limitations identified: Feed can also be a source of contaminants (mycotoxins, xenobiotics, pathogens). Organic systems cannot use synthetic amino acids. Search for alternative protein sources to imported soya bean. |
||
Method of presentation |
Increase particle size to reduce the risk of ulcers (P) |
P1 |
Distribute long-stemmed dry fodder to reduce digestive disorders (L) |
P1 |
|
Provide dry fodder in the form of compressed blocks to allow animals to gnaw (L) |
P1 |
|
Make plants or extracts freely available in the building or on the run (L, V) |
P1 |
|
Method and schedule of distribution and management of feed transitions |
Restrict feed after weaning to reduce the frequency of digestive disorders (L) |
P1 and P2 |
Feed before weaning to stimulate the development of digestive capacity and the establishment of microbiota (P, L) |
P1 and P2 |
|
Switch from free-feeding to meals in order to prepare animals for force-feeding (V) |
P1 |
|
Limitations identified: Risk of female weight loss: only one feeder for females and young rabbits (L) |
||
Individualisation |
Perform precision feeding to reduce excessive fattening or breaks in homeostasis (P, L, V) |
P1 |
a Principles of integrated animal health management (section 1). P1: avoid risk situations and contact with pests; P2: resist or tolerate pests
b the feed is a medium for administering substances that benefit animal health
Table 6. Practices for integrated animal health management of monogastric animals (Gotti et al., 2021b).
Practices |
|---|
Acquire knowledge |
Learn more about the biology, physiology and behaviour of animals |
Identify good/poor physical health and welfare of animals |
Learn more about animal diseases |
Improve knowledge of biosafety and prophylaxis rules |
Train in animal handling |
Train in the well-reasoned use of antibiotics |
Train in the detection of diseases and health risks in animals |
Use the skills of a work group and experts |
Consult regularly with external professionals |
Develop the knowledge of the entire work group |
Develop a strategy for distributing responsibilities within the work group |
Communicate within the animal-health work group |
Acquire information on the health of animals and herds |
Develop an information and data-recording system on the health of the herd |
Observe animals and obtain tools to supplement observation |
Obtain equipment to record farm performance and detect the onset of disorders |
Organise equipment and work |
Optimise the organisation of work on the farm to maximise compliance with biosecurity rules |
Obtain equipment or optimise the organisation of work on the farm to have time to observe the health of the animals and improve the quality of relationships with the animals |
Integrate into networks |
Provide support for risk-taking during changes that lead to reconfiguration of the system |
Provide economic support for productivity losses caused by de-intensification of animal production |
3.2. Time frames of practices that can be used in animal production
The practices used for integrated animal health management can act over different time frames (Figure 3). Thus, practices be distinguished by i) direct effects: e.g. feeding protein to piglets around weaning influences their digestive health (Liao, 2021); ii) delayed effects: e.g. egg incubation temperature modifies the heat tolerance of chickens at the end of rearing (Loyau et al., 2015), or the consumption of essential oils during chick start-up permanently modifies the transcriptome of chickens depending on their postnatal experience (Foury et al., 2020) and iii) effects transmitted between generations: supplementing maternal diets of ducks with omega-3 fatty acids can reduce the phenomenon of pecking in the offspring (Baéza et al., 2017).
Figure 3. Time frames of practices that influence animal health: direct effects (A), delayed effects (B) and effects transmitted between generations (C).

Considering the time frames of practices encourages a long-term vision of animal health management and better understanding of the connections among the systems within the entire production system or between the links in a production chain (breeding stock vs. growing stock; hatchery vs. farm practices). For example, in poultry farming, Van der Waaij et al. (2011) recommend similar rearing conditions for breeders and their offspring. However, in the current broiler industry, breeders are fed a restricted diet to maximise their reproductive performance, while their offspring are fed ad libitum to maximise their growth. Restricting the feed of breeders influences their welfare and immune status (Decuypere et al., 2010) and limits the growth of their offspring (Bowling et al., 2018).
4. Integrating the practices into monogastric animal health in current systems
The practices available at the farming system level for integrated health management of monogastric animals should not be considered separately, but together in an approach of integrated health management. Integration means that practices are coordinated to build up animal health to achieve and/or maintain balance. This includes coordinating practices of prevention and those that develop the adaptive capacity of animals. Conversely, health disorders, when not specific to a pathogen, as in the case of production diseases, are generally multifactorial in origin and occur because several elements combine to exceed the adaptive capacity of the animals (Table 7). In the sections below, we describe how this coordination is performed on pig, poultry and rabbit farms. Note that some of the practices used for their beneficial effects on animal health also help maintain productivity.
Table 7. Multifactoriality of practices by category that influence the emergence of frequent health problems in rabbit (A), pig (B) or poultry (C and D) farming. The five categories of practices are described in Tables 1 to 5.
Disorder |
Influential practices: living environment; reproduction; herd management; animals; feeding |
|---|---|
A. Digestive disorders in young rabbits |
Age at weaning; Genetic type of animals; Fibre intake; Control of intake; Dietary transition |
B. Digestive disorders in piglets |
Comfort (floor and temperature); Age at weaning; Feed composition, including protein intake |
C. Myopathies in broiler chickens |
Area available; Animal density; Age at slaughter; Genetic type of animals; Energy content of the feed |
D. Pecking in laying hensa |
Lack of enrichment; Bedding; Animal density; Genetic type of animals; Feed restriction |
a Interactions between practices are possible. For example, for myopathies in broiler chickens, the age at slaughter and energy content of the feed have a strong influence only on fast-growing genetic lines.
4.1. Weaning management in pig farming
In conventional pig farming, piglets are weaned at 3-4 weeks of age, when their digestive and immune systems are still immature. Separation from the mother, the transition from milk to solid plant feed and a new microbial and social environment contribute to destabilise piglet physiology and can lead to digestive disorders. Several practices can reduce the onset and/or severity of these disorders: i) later weaning; ii) creep feeding; iii) adequate access to drinking water during lactation; iv) using a palatable post-weaning feed, low in protein and balanced in essential amino acids, that contains highly digestible sources of protein and energy, as well as fibre for the development of the intestinal microbiota, along with additives (probiotics, organic acids, clay, etc.) to maintain digestive physiology and microbiota (Pluske et al., 2018); v) housing that promotes thermal comfort for the animals, cleaning and disinfecting the housing, and regulating the movement of personnel on the farm to reduce the presence and transmission of pathogenic bacteria (Corrégé and Hémonic, 2018) and vi) vaccination of piglets against the main pathogens responsible for infectious diarrhoea (Melkebeek et al., 2013).
4.2 Consistency between systems on a rabbit farm
In rabbit farming, various practices are used in a consistent manner with the four categories of animals present (suckling rabbits, growing rabbits, future breeding females, breeding females) to achieve integrated animal health management.
After kindling, farmers homogenise litter size by eliminating offspring with a live weight less than 35 g and by cross-fostering to equalise litter size. This strategy reduces competition between young rabbits for access to milk and improves their thermoregulation (access to nest warmth), which promotes their survival and development (Rödel et al., 2008). It also helps the mothers to maintain an adequate body condition (weight is lost if litters are supernumerary), which increases their longevity. The feeding strategy for females focuses on managing the trade-off between meeting important nutritional needs during lactation (energy-rich feed at the beginning of lactation) and maturation of the digestive system of the young rabbits (high-fibre, low-starch feed before weaning; Gidenne and Fortun-Lamothe, 2002). In addition, young females and breeding females are vaccinated against VHD and myxomatosis. After weaning, feeding high-fibre diets (Gidenne et al., 2010) and controlling intake (Gidenne et al., 2012) reduce the occurrence of non-specific digestive disorders in growing rabbits. These strategies are combined with strict hygiene protocols at the entrance to the rearing rooms and regular thorough cleaning, which is made possible by the batched system. In addition, the batched system allows litters to be kept after weaning, which reduces social stress. Future breeding females generally arrive at 1-2 days of age on commercial farms and are adopted by existing breeding females, which allows them to adapt to the rearing conditions (environmental conditions, microbial environment, etc.) and reduces the entry of pathogens onto the farm, particularly Pasteurella multocida, the pathogen responsible for the main respiratory disease in females (Coudert et al., 1999). In addition, to manage pasteurellosis in breeding rabbits (respiratory and abscess forms), control of environmental conditions (temperature, humidity and air velocity) is combined with the elimination of animals that show clinical symptoms to prevent spread of the disease on the farm. Animals resistant to this highly problematic pathogen can be selected (Shrestha et al., 2020), but this is rarely done in breeding centres.
4.3. Diversification of systems in the poultry sector
In poultry farming, infectious diseases are managed using several complementary practices. Animals are vaccinated to protect them against infectious bronchitis and Marek's and Gumboro diseases (broiler chickens), or salmonella and coccidia (laying hens). Strictly complying with biosecurity rules (disinfection and control of entries and exits on the farm), eliminating sick animals and disinfecting after each flock aims to reduce the entry and concentration of pathogens on the farm. In addition, controlling ventilation, humidity and temperature in the building and keeping bedding clean and dry helps to reduce damage to the animals' legs (pododermatitis, tarsal burns, etc.). Poultry farms invest strongly in practices that support the animals' ability to adapt to environmental variations that may be stressful and influence health. This means using feeds with a high value for health (micronutrients, antioxidants, probiotics, plant extracts, essential oils, etc.) while maintaining the expected productivity of poultry (Bhagwat et al., 2021; Abd El-Hack et al., 2020).
However, total confinement in small housing units without environmental enrichment and high animal densities (up to 42 kg/m2 in broiler production) have been strongly criticised for their negative effects on animal welfare. Thus, enriching the living environment is an essential practice to reduce aggression between animals (Rodenburg et al., 2013). However, this practice is not always sufficient to keep the animals healthy, and this type of farming is currently criticised for not considering animal welfare sufficiently. Consequently, many alternative systems have been developed recently. Animal access to a run can diversify the behavioural repertoire (psychosocial component of health), and selecting lines with a low growth rate can reduce musculoskeletal disorders, including muscular myopathies. In these alternative systems, animals are exposed more to parasites and pathogens transmitted by wildlife. In addition, using mobile buildings could reduce disease pressure on the run, as could the planting of medicinal plants. Phytotherapy and aromatherapy could be used more generally in feed or drinking water (Travel et al., 2021) or via self-medication with plants grown on the run or provided in buildings (Guilloteau et al., 2019; Foury et al., 2020). However, access to runs may also be temporarily prohibited to protect animal health, for example during avian influenza episodes. Finally, intermediate systems, which provide animals access to winter gardens or fenced or netted areas, are being developed to provide animals with space to express their behavioural and social needs with little exposure to environmental pathogens or contamination by wildlife.
5. Limitations of current strategies and ways forward
Generally, over the past few decades, health management on monogastric animal farms has focused on the physical health of the animals and the desire to increase animal productivity for economic reasons. Certain technical choices now cause health and welfare problems (e.g. housing in wire cages and pododermatitis of breeding rabbits). Moreover, these choices have often been made to the detriment of the psychosocial health of the animals (e.g. high animal density, inappropriate living environments and pecking for laying hens; limiting the behavioural repertoire of rabbits reared in cages; stereotypies at the end of pregnancy in sows housed in boxes).
Below, we discuss limitations for pig, rabbit and poultry farms as well as potential strategies for technical development. These limitations are not only technical, but also human and social (need for security, support for new practices, competition for working time), economic (investments, farm profitability) or scientific (genetic selection, early diagnosis of disorders; Ducrot et al., 2018; Piel et al., 2019).
In conventional pig farming, managing piglet health at weaning without using antibiotics remains difficult on some farms. One reason is the age at weaning. European Union Directive 2008/120/EC issued minimum standards for the protection of pigs, which recommends that no piglet should be separated from its mother before it is 28 days old. However, the practice of weaning at 21 days of age has developed strongly in France in recent years to encourage a rapid return to heat and maximise the numerical productivity of sows. Studies in the Americas (Faccin et al., 2020) and Europe (Postma et al., 2017) have shown that a later weaning age (35 days) is associated with lower antibiotic use, probably due to greater maturity of the piglets. Other avenues for progress are mentioned, but they represent significant scientific and technical challenges. They include i) providing forage, which in addition to getting the animals accustomed to solid feed and promoting the development of microbiota, provides a substrate for play, ii) managing sows to promote their welfare during gestation and iii) mixing piglets before weaning to promote social interactions between piglets from different litters (Blavi et al., 2021).
Delaying the weaning age is encouraged in organic farming, which requires weaning after 42 days to approximate natural conditions (90-120 days), and provides interesting avenues for improving piglet health without altering the sows' return to reproduction (Ferchaud et al., unpublished data). Using animals that are resistant to the main pathogens involved in diarrhoea, resilient to weaning, efficient from a feeding viewpoint and adapted to varied and variable rearing conditions would be a major advance (Le Roy et al., 2019). Implementing this depends on scientific advances and changes in breeding programmes. Finally, current housing conditions for lactating sows and piglets are not always conducive to the expression of natural behaviours, positive mother-young relationships, learning to feed or interactions between piglets from different litters. Changing housing systems is often hindered by the economic cost of investment (Bertin and Ramonet, 2016).
In rabbit farming, housing animals in cages with wire mesh floors is justified to manage parasitic infestation by reducing the animals’ contact with their faeces. However, coccidia are present on most conventional farms (protozoa of the genus Eimeria; Licois, 2009) and the use of anticoccidial medicines, mainly robenidine®, remains frequent and is currently causing resistance problems. At the same time, this housing method leads to pododermatitis in breeding females (prevalence: 5-15%; Rosell and De la Fuente, 2013) despite the presence of plastic "leg rest" bottoms. For this reason, rabbit farming is moving towards rearing growing rabbits in pens, often with slatted floors, and in large groups (more than 20 animals). This change leads to more social interactions (at least with conspecifics), which can modify the dynamics of pathogen transmission, especially if animal density is not reduced (currently limited to 45 kg/m² at 70 days of age for growing rabbits). It is thus necessary to reconsider i) the layout of the living environment, to not disrupt these social interactions in relation to animal expectations, and ii) implementation of hygiene and prophylaxis protocols, to reduce pathogen transmission within these large groups. Changing housing could prevent the maintenance of multi-purpose housing for breeding and growing rabbits and lead to abandonment of the “all-full-all-empty” health management currently practised on half of French rabbit farms. This type of management has the advantage of allowing the building and housing to be cleaned and disinfected after each batch of animals. However, it can also promote pathogen transmission because the animals in this confined environment all have the same physiological stage and are therefore exposed to the same degree of risk. Furthermore, as the microbiota influences maturation of the immune system, excessive hygiene could ultimately be detrimental to animal health. Combining animals at different physiological stages is a practice that predates the batch system and should be studied considering the new production methods.
For breeding females, respiratory problems persist despite the methods used to control environmental conditions. Using resistant animals seems to be the most promising approach but remains to be implemented. Furthermore, it has been shown that reducing the rate of reproduction (e.g. insemination every seven weeks instead of the current six weeks) reduces the nutritional demands of the females, which improves their body condition and increases their longevity. Nevertheless, this practice is not widespread and is often restricted to summer for economic reasons (reduction in annual productivity). Finally, individual housing of females is contested by animal rights activists because rabbits are social animals that live in colonies. Group housing of familiar females (biological or milk sisters) is possible until the first kindling (Laclef et al., 2021). After that, however, female rabbits fight and seriously injure each other in an attempt to establish a social hierarchy. A system that is a satisfactory compromise among reproductive performance, health and behavioural expression remains to be found.
In poultry farming, a major limitation to animal health and welfare is the segmentation of the production chain (breeder rearing, hatchery, chicken or pullet rearing, laying house for egg production systems, transport to the slaughterhouse). It leads to waiting and transport phases, especially between the hatchery and the farm, that are a source of considerable stress for the animals. After being handled (sorting and sexing), they are kept without water and feed for several hours and, during transport, may be subjected to variations in temperature and humidity, as well as vibrations and transport hazards. This has been shown to have a lasting effect on their subsequent physical (postnatal hypothermia, altered metabolism, growth retardation; Guilloteau et al., 2019, Beauclercq et al., 2019; Foury et al., 2020) and psychosocial (Hollemans et al., 2018) health. Access to water and feed at hatching is necessary, and they can be provided in hydrated feed at the hatchery and/or in transport boxes or by hatching on-farm (Van de Ven et al., 2011; Leterrier, unpublished data). Furthermore, early implantation of a natural (from or in the presence of adult hens) or selected (gut microflora, probiotics) microbiota barrier reduces the load of salmonella and other enteropathogens (Schneitz, 2005), facilitates maturation of the immune system and helps decrease the mortality rate, which remains high in the start-up phase.
Using highly specialised genetic lines whose metabolism is oriented mainly towards the targeted biological function (growth or egg production) also influences animal health. For example, laying hens that are selected for egg production have a weakened skeleton after 70 weeks. This situation is exacerbated by the rearing conditions, which do not provide the animals with sufficient opportunities to move. In contrast, chickens that are selected for rapid growth and high feed efficiency have pectoral muscle yields greater than 20% but a weakened thermoregulatory capacity (Piestun et al., 2008) and are prone to frequent muscle myopathies such as white streaks, wooden breast or spaghetti muscle (Praud et al., 2020). Using slower-growing lines or mixed lines for both egg and meat production could prevent these disorders.
Finally, feather pecking in laying hens remains a major health problem that is currently managed by modifying the animal's physical integrity (beak trimming). This practice could be avoided by providing fibre-rich feed, enriching the living environment (adapted litter, pecking stones or blocks), genetic selection (control of fear and stress levels) and reducing rearing densities (Rodenburg et al., 2013; Zepp et al., 2018).
6. Future challenges
Currently, some European consumers want animal welfare to be considered better. They do not support practices that modify physical integrity (castration without anaesthesia, tooth grinding, beak trimming, etc.), disapprove of animal production in cages and want animals to be allowed to move freely. Following the citizens' initiative "End the Cage Age" (https://www.endthecageage.eu/), the European Commission has committed to developing a framework for legislative change that would require ending cage farming by the end of 2023 and that would come into force in 2027. Monogastric animal farms are particularly targeted by this initiative (breeding animals and growing rabbits, etc.).
Monogastric animal farming systems will have to change significantly, and health management will have to be re-evaluated accordingly. Improved welfare considerations, however, can sometimes have a negative influence on animal health in these farming systems. This is the case for rabbits (Szendrö et al., 2019) or sows kept in groups. Giving them the opportunity to interact socially with conspecifics results in frequent fighting and a high injury rate until the social hierarchy is established. Research has also shown that although animals with access to the outdoors appear to be subject to fewer respiratory diseases than animals kept in confined systems, they are subject to higher perinatal and reproductive mortality (Delsart et al., 2020). This is due mainly to inadequate control of light and thermal conditions. Health management in these systems should thus include ways to help animals adapt to climate variations (windbreaks, shelter, refuge; Skuce et al., 2013), the ability to predict these variations (access to data or weather alerts) to be able to shelter the animals beforehand and improving the layout of outdoor areas (trees, windbreaks, protective barriers, etc.). Furthermore, animals with access to the outdoors are exposed to more parasites and other infectious agents not encountered in buildings (via feed, soil and contact with wildlife). The layout of the living environment or innovative practices will have to be considered to manage these new challenges. The experiences of farms that comply with organic farming specifications could be valuable (Vaarst and Alroe, 2012). These changes are likely to increase the diversity of farming systems and variability in the health and welfare of animals. These changes are also likely to require more individualised ways to monitor and manage behaviour and health parameters that could benefit from digital applications (Grosse-Kleimann et al., 2021).
Conclusion
Animal health, which is both a state and a process, is built up throughout an animal’s life. In pig, rabbit and poultry farming, many practices are available and must be used consistently in an approach of integrated animal health management. Their main objective is to promote health, prevent contact with harmful biotic and abiotic elements, and support the adaptive capacity of animals so they become tolerant of these elements. However, antibiotics and antiparasitic agents are still used on these farms. This situation is due mainly to technical or organisational choices that are motivated by economic reasons, which lead to situations that exceed the animals' capacity to adapt. This is the case in pig farming, in which the digestive and immune immaturity of piglets at weaning is a risk factor for digestive disorders. This is also the case in poultry farming, in which the conditions under which the animals are transported from the hatchery to the farm generate stress that has a lasting effect on their health, and in which genetic selection that is oriented toward production weakens their ability to adapt.
Some of these choices, especially those related to housing, are currently criticised by society for not respecting animal welfare. These criticisms are driving the emergence of new farming systems that raise new questions about animal health. Animals' access to the outdoors also modifies the boundaries and dynamics of flows (matter, xenobiotics, pathogens, individuals, genes) between animals and wildlife and between animals and highly human-modified environments. In reference to the "One Health" concept, greater proximity of animals to vectors, pathogen reservoirs or xenobiotics in the wild or to human-modified environments represents a challenge for integrated health management (Esther et al., 2016).
Acknowledgements
The authors thank Catherine Schouler for her contribution to the reflections and her proofreading, Cécile Berri for her expert contribution on avian muscle disorders and Agnès Girard for her help with bibliographic research.
The manuscript was translated by DeepL and proofread by Mme Corson.
References
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y.A., Batiha G.E., Khafaga A.F., Abdel-Moneim A.E., Alagawany M., 2020. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr., 104, 1835-1850. doi:10.1111/jpn.13454
- Anses, 2020. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2019, Anses-ANMV, France, novembre 2020, rapport, 97p. https://www.anses.fr/system/files/ANMV-Ra-Antibiotiques2019.pdf consulté le 04/08/2021
- Baéza E., Chartrin P., Bordeau T., Lessire M., Thoby J.M., Gigaud V., Blanchet M., Alinier A., Leterrier C., 2017. Effet des acides gras Polyinsaturés N-3 apportés au cours du développement embryonnaire et des périodes de démarrage et croissance sur le comportement de picage des canards de Barbarie. Journées Rech. Avicoles, 12, 423-427.
- Beauclercq S., Lefèvre A., Montigny F., Collin A., Tesseraud S., Leterrier C., Emond P., Guilloteau L.A., 2019. A multiplatform metabolomic approach to characterize fecal signatures of negative postnatal events in chicks: a pilot study. J. Anim. Sci., Biotechnol., 9, 21. doi:10.1186/s40104-019-0335-8
- Berry R., López-Martínez G., 2020. A dose of experimental hormesis: When mild stress protects and improves animal performance. Comparative Biochemistry and Physiology Part A: Mol. Integr. Physiol., 242, 110658. doi:10.1016/j.cbpa.2020.110658
- Bertin C., Ramonet Y., 2016. État des lieux des bâtiments d’élevage de porcs en Bretagne chez les naisseurs-engraisseurs en 2015. Journées Rech. Porcine, 48, 1-7.
- Bhagwat V.G., Balamurugan E., Rangesh P., 2021.Cocktail of chelated minerals and phytogenic feed additives in the poultry industry: A review. Vet. World., 4, 364-371. doi:10.14202/vetworld
- Blanc F., Ollion E., Puillet L., Delaby L., Ingrand S., Tichit M., Friggens N., 2013. Évaluation quantitative de la robustesse des animaux et du troupeau : quels principes retenir ? Renc. Rech. Rum., Paris, France, 20, 365-272.
- Blanco-Penedo I., Fernández González C., Tamminen L.M., Sundrum A., Emanuelson U., 2018. Priorities and Future Actions for an Effective Use of Phytotherapy in Livestock—Outputs from an Expert Workshop. Front. Vet. Sci., 22. doi:10.3389/fvets.2017.00248
- Blavi L., Solà-Oriol D., Llonch P., López-Vergé S., Martín-Orúe S.M., Pérez J.F., 2021. Management and feeding strategies in early life to increase piglet performance and welfare around weaning: A Review. Animals, 11, 302. doi:10.3390/ani11020302
- Bowling M., Forder R., Hughes R.J., Weaver S., Hynd P.I., 2018. Effect of restricted feed intake in broiler breeder hens on their stress levels and the growth and immunology of their offspring. Transl. Anim. Sci., 2, 263-271. doi:10.1093/tas/txy064
- Brambell F.W.R., 1970. The Transmission of Passive Immunity from Mother to Young. Amsterdam: North-Holland Research Monographs Frontiers of Biol. 18, 385p.
- Chatelet A., Gondret F., Merlot E., Gilbert H., Friggens N.C., Le Floc'h N., 2018. Impact of hygiene of housing conditions on performance and health of two pig genetic lines divergent for residual feed intake. Animal, 12, 350-358. doi:10.1017/S1751731117001379
- Corrégé I., Hémonic A., 2018. La biosécurité en élevage de porcs : enjeux, observance, freins et perspectives de progrès. Journées Rech. Porcine, 50, 177-188.
- Coudert P., Rideaud P., Kpodékon M., 1999. Le point sur les pasteurelloses du lapin : Rapport de synthèse. Journées Rech. Cunicole, 8, 3-12 Paris, France,
- Darré J.P., Mathieu A., Lasseur J., 2004. Le sens des pratiques. Conceptions d'agriculteurs et 43 modèles d'agronomes. Inra Éditions, Coll. Science Update, 320p.
- Decuypere E., Bruggeman V., Everaert N., Li Y., BoonenR., De Tavernier J., Janssens S., Buys N., 2010. The broiler breeder paradox: ethical, genetic and physiological perspectives, and suggestions for solutions. Br. Poultry Sci., 51, 569-579. doi:10.1080/00071668.2010.519121
- Dedieu B., Ingrand S., 2010. Incertitude et adaptation : cadres théoriques et application à l’analyse de la dynamique des systèmes d’élevage. INRA Prod. Anim., 23, 81-90. doi:10.20870/productions-animales.2010.23.1.3289
- Dedieu B., Faverdin P., Dourmad J.Y., Gibon A., 2008. Système d'élevage, un concept pour raisonner les transformations de l'élevage. INRA Prod. Anim., 21, 45-58. doi:10.20870/productions-animales.2008.21.1.3374
- Delsart M., Pol F., Dufour B., Rose, N., Fablet C., 2020. Pig Farming in Alternative Systems: Strengths and Challenges in Terms of Animal Welfare, Biosecurity, Animal Health and Pork Safety. Agriculture-Basel, 10, article 261. doi:10.3390/agriculture10070261
- Döring T.F., Vieweger A., Pautasso, M., Vaarst, M., Finkh, M.R., Wolfe, M.S., 2015. Resilience as a universal criterion of health. J. Sci. Food Agric., 95, 455-465. doi:10.1002/jsfa.6539
- Ducarmon Q.R., Zwittink R.D., Hornung B.V.H., van Schaik W., Young V.B., Kuijper E.J., 2019. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev., 83, e00007-19. doi:10.1128/MMBR.00007-19
- Ducos A., Douhard F., Savietto D., Sautier M., Fillon V., Gunia M., Rupp R., Moreno-Romieux C., Mignion-Grasteau S., Gilbert, H., Fortun-Lamothe L., 2021. Contributions de la génétique animale à la transition agroécologique des systèmes d’élevage. INRAE Prod. Anim., 34, 79-96. doi:10.20870/productions-animales.2021.34.2.4773
- Ducrot C., Adam C., Beaugrand F., Belloc C., Bluhm J., Chauvin C., Cholton M., Collineau L., Faisnel J., Fortané N., Hellec F., Hémonic A., Jloy N., Lhermie G., Magne M.A., Paul M., Poizat A., Raboisson D., Rousset N., 2018. Apport de la sociologie à l’étude de la réduction d’usage des antibiotiques. INRAE Prod. Anim., 31, 307-324. doi:10.20870/productions-animales.2018.31.4.2395
- Dumont B., Fortun-Lamothe L., Jouven M., Thomas M., Tichit M., 2013. Prospects from agroecology and industrial ecology for animal production in the 21st century. Animal, 7, 1028-1043. doi:10.1017/S1751731112002418
- Durand D., Collin A., Merlot E., Baéza E., Guilloteau L.A., Le Floc’h N., Thomas A., Fontagné-Dicharry S., Gondret F., 2021. Review: Implication of redox imbalance in animal health and performance at critical periods, insights from different farm species. Animal, 16, 100543. doi:10.1016/j.animal.2022.100543
- Faccin J.E.G., Tokach M.D., Allerson M.W., Woodworth J.C., DeRouchey J.M., Dritz S.S., Bortolozzo F.P., Goodband R.D., 2020. Relationship between weaning age and antibiotic usage on pig growth performance and mortality. J. Anim. Sci., 98, 1-10. doi:10.1093/jas/skaa363
- Falker-Gieske C., Mott A., Preu S., Franzenburg S0, Bessei W0, Bennewitz J., Tetens J., 2020. Analysis of the brain transcriptome in lines of laying hens divergently selected for feather pecking. B.M.C. Genomics, 21, 595. doi:10.1186/s12864-020-07002-1
- Foong J.P.P, Hung L.Y., Poon S., Savidge T.C., Bornstein J.C., 2020. Early life interaction between the microbiota and the enteric nervous system. Am. J. Phys. Gastro. Liver Phys., 319, G541-G548. doi:10.1152/ajpgi.00288.2020
- Fortun-Lamothe L., Prunier A., Bolet G., Lebas F., 1999. Physiological mechanisms involved in the effects of concurrent pregnancy and lactation on fœtal growth and survival in the rabbit. Livest. Prod. Sci., 60, 229-241. doi:10.1016/S0301-6226(99)00096-2
- Fortun-Lamothe L., Collet P.S., Read A.K., Mariana J.C., 2000. Effects of concurrent pregnancy and lactation in rabbit does on the growth of follicles in daughters’ ovaries. World Rabbit Sci. 8, 33-40. doi:10.4995/wrs.2000.415
- Fortun-Lamothe L., Combes S., Balmisse E., Collin A., Ferchaud S., Germain K., Pinard-Van Der Laan M. H., Schouler C., Le Floc'h N., 2017. A conceptual framework to promote integrated health management in monogastrics. In Proc. 68th EAAP Ann. Meet., Tallinn, Estonia, 28 August to 1 September, p245.
- Foury A., Collin A., Helbling J.C., Leterrier C., Moisan M.P., Guilloteau L.A., 2020. Spontaneous intake of essential oils after a negative postnatal experience has long-term effects on blood transcriptome in chickens. Sci. Rep. 10, 20702. doi:10.1038/s41598-020-77732-5
- Fraser D., Duncan I.J., Edwards S.A., Grandin T., Gregory N.G., Guyonnet V., Hemsworth P.H., Huertas S.M., Huzzey J.M., Mellor D.J., Mench J.A., Spinka M., Whay H.R., 2013. General Principles for the welfare of animals in production systems: the underlying science and its application. Vet. J., 198, 19-27. https://doi/10.1016/j.tvjl.2013.06.028
- Friggens N.C., Blanc F., Berry D.P., Puillet L., 2017. Review: Deciphering animal robustness. A synthesis to facilitate its use in livestock breeding and management. Animal, 11, 2237-2251. doi:10.1017/S175173111700088X
- Ganiere J.P., Andre-Fontaine G., Drouin P., Faye B., Madec F., Rosnere G., Fourichon C., Wang B. Tillon J.P., 1991. L’écopathologie : une méthode d’approche de la santé en élevage. INRA Prod. Anim., 4, 247-256. doi:10.20870/productions-animales.1991.4.3.4339
- Gidenne T., Fortun-Lamothe L., 2002. Feeding strategy for young rabbits around weaning: A review of digestive capacity and nutritional needs. Anim. Sci., 75, 169-184. doi:10.1017/S1357729800052942
- Gidenne T., García J., Lebas F., Licois D., 2010. Nutrition and feeding strategy: interactions with pathology. In book: Nutrition of the Rabbit. de Blas C., Wiseman J. (Eds). 10, 179-199 CAB International. doi:10.1079/9781845936693.0179
- Gotti V., Manoli C., Dedieu B., 2021a. Work organization and integrated management of animal health: What connections do they have? ISWA, 2nd Intern. Symp. Work in Agriculture: Thinking the future of work in agriculture, Clermont-Ferrand, France.
- Gotti V., Manoli C., Dedieu B., 2021b. Exploration of health practices in dairy farms: links with the farming system and work organization. 72nd Ann. Meeting Eur. Fed. Anim. Sci., (EAAP), Davos, Switzerland.
- Grosse-Kleimann J., Plate H., Meyer H., Gerhardy H., Heucke C.E., Kreienbrock L., 2021. Health monitoring of finishing pigs by secondary data use - a longitudinal analysis. Porcine Health Manag., 7. doi:10.1186/s40813-021-00197-z
- Guilloteau L.A., Collin A., Koch A., Leterrier C., 2019. Spontaneous Intake and Long-Term Effects of Essential Oils After a Negative Postnatal Experience in Chicks. Front. Vet. Sci., 6. doi:10.3389/fvets.2019.00072
- Gunia M., David I., Hurtaud J., Maupin M., Gilbert H., Garreau H., 2018. Genetic parameters for resistance to non-specific diseases and production traits measured in challenging and selection environments; Application to a Rabbit Case. Front. Genet., 9, 467. doi:10.3389/fgene.2018.00467
- Gunnarsson S., 2006. "The conceptualisation of health and disease in veterinary medicine." Acta Vet. Scand., 48, 20. doi:10.1186/1751-0147-48-20
- Halfon N., Larson K., Lu M., Tullis E., Russ E., 2014. Lifecourse Health Development: Past, Present and Future. Matern. Child Health J., 18, 344-365. doi:10.1007/s10995-013-1346-2
- Hellec F., Manoli C., De Joybert M., 2021. Alternative medicines on the farm: a study of dairy farmers' experiences in France. Front Vet. Sci., 8, n° 563957. doi:10.3389/fvets.2021.563957
- Hertzman C., Power C., 2011. Health and human development: Understandings from life-course research. Dev. Neuropsy., 719-744. doi:10.1080/87565641.2003.9651917
- Hickman R.A., Leangapichart T., LunhaK., Jiwakanon J., AngkititrakulS., MagnussonU., SundeM., JarhultJ.D., 2021. Exploring the Antibiotic Resistance Burden in Livestock, Livestock Handlers and Their Non-Livestock Handling Contacts: A One Health Perspective. Front. Microbiol., 12, n°651461. doi:10.3389/fmicb.2021.651461
- Hieke A.C., Hubert S.M., Athrey G., 2019. Circadian disruption and divergent microbiota acquisition under extended photoperiod regimens in chicken. PeerJ., 7, e6592. doi:10.7717/peerj.6592.
- Hollemans M.S., de Vries S., Lammers A., Clouard C., 2018. Effects of early nutrition and transport of 1-day-old chickens on production performance and fear response. Poultry Sci., 97, 2534-2542. doi:10.3382/ps/pey106
- Jiang X., Lu N., Zhao H., Yuan H., Xia D., Lei H., 2020. The Microbiome-Metabolome Response in the Colon of Piglets Under the Status of Weaning Stress. Front Microbiol., 11, 2055. doi:10.3389/fmicb.2020.02055
- Laclef E., Savietto D., Warin L., Huang Y., Bonnemère J.M., Combes S., Gidenne T., Fortun-Lamothe L., 2021. Part-time group housing if familiar rabbit does in large partitioned space: effects on performance and behaviour. 12th World Rabbit Congress, 3-5 November 2021, Nantes, France.
- Lanning D., Sethupathi P., Rhee K.J., Zhai S.K., Knight K.L., 2000. Intestinal microflora and diversification of the rabbit antibody repertoire. J. Immunol., 165, 2012-2019. doi:10.4049/jimmunol.165.4.2012
- Le Floc'h N., Boudon A., Montagne A., Gilbert H., Gondret F., Lebret B., Lefaucheur L., Louveau I., Merlot E., Père M.C., Meunier-Salaün M.C., Prunier A., Quesnel H., 2021. Santé et bien-être de la truie gestante et du porc en croissance. INRAE Prod. Anim., 34, 211-226. doi:10.20870/productions-animales.2021.34.3.4879
- Le Roy P., Ducos A., Phocas F., 2019. Quelles performances pour les animaux de demain ? Objectifs et méthodes de sélection. INRAE Prod. Anim., 32, 233-246. doi:10.20870/productions-animales.2019.32.2.2466
- Lhermie G., Raboisson D., Krebs S., Dupraz P., 2015. Facteurs déterminants et leviers de réduction de l’usage des antibiotiques en productions animales. Economie rurale, 348. doi:10.4000/economierurale
- Liao S.F., 2021. Maintain or Improve Piglet Gut Health around Weanling: The Fundamental Effects of Dietary Amino Acids. Animals, 11, 1110. doi:10.3390/ani11041110
- Licois D., 2009. Pathologie d’origine bactérienne et parasitaire chez le lapin : apports de la dernière décennie. In Journées Rech. Cunicole, 13, 17-18 novembre 2009. Le Mans, France
- Loyau T., Bedrani L., Berri C., Métayer-Coustard S., Praud C., Coustham V., Mignon-Grasteau S., Duclos M.J., Tesseraud S., Rideau N., Hennequet-Antier C., Everaert N., Yahav S., Collin A., 2015. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: a review. Animal, 9, 76-85. doi:10.1017/S1751731114001931
- Lyte M., 2013. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog., 9, e1003726. doi:10.1371/journal.ppat.1003726
- Madderm J.G., Walkers J., Van Rooyen D., Knobel E., Vandamme E., Berkvens D., Vanwambeke S.O., De Clerc E.M., 2012. e-Surveillance in Animal Health: use and evaluation of mobile tools. Parasitol., 139, 1831-1842. https://doi/10.1017/S0031182012000571
- Manoli C., Gambara T., Di Bianco S., Bareille N., Kaufmann P., Dufay-Lefort A.C., Poissonnet A., Wache A., 2021. How are animal health monitoring tools used? The point of view of french livestock farmers. 72nd Ann. Meet. Europ. Fed. Anim. Sci., (EAAP), 30th August - 3rd September 2021, Davos, Switzerland.
- Melkebeek V., Goddeeris B.M., Cox E., 2013. ETEC vaccination in pigs. Vet. Immunol. Immunopathol., 152, 37-42. https://doi/10.1016/j.vetimm.2012.09.024
- Menjon P., d’Orgeval R., 1983. Entre atelier et filière : le système d’élevage. Agriscope, I (1), « L’exploitation agricole, une approche globale », 42-53.
- Nilsson E.E., Sadler-Riggleman I., Skinner M.K., 2018. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics, 4, doi:10.1093/eep/dvy016
- Paul M., Belloc C., Rousset N., Hemonic A., Margueries J., Le Coz P., Le Normand B., Hercule J., Roguet C., Leblanc-Maridor M., Chauvin C., Ducrot C., 2021. Évolution de l’usage des antibiotiques en filières monogastriques : état d’avancement et perspectives. INRAE Prod. Anim., 32, 291-304. doi:10.20870/productions-animales.2019.32.2.2485
- Piel Y., Le Gall A., Belloc C., Leblanc-Maridor M., 2019. Pratiques et perceptions de l’usage des antibFacciotiques chez les éleveurs porcins. Journées Rech. Porcine, 51, 283-288.
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S., 2008. Thermal manipulations during broiler embryogenesis: Effect on the acquisition of thermotolerance. Poultry Sci., 87, 1516-1525. doi:10.3382/ps.2008-00030
- Pluske J.R., Turpin D.L., Kim J.C., 2018. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr., 4,187-196. doi:10.1016/j.aninu.2017.12.004
- Postma M., Vanderhaeghen W., Sarrazin S., Maes D., Dewulf J., 2017. Reducing Antimicrobial Usage in Pig Production without Jeopardizing Production Parameters. Zoonoses Public Health, 64, 63-74. doi:10.1111/zph.12283
- Praud C., Jimenez J., Pampouille E., Courousse N., Godet E., Le Bihan-Duval E., Berri C., 2020. Molecular Phenotyping of White Striping and Wooden Breast Myopathies in Chicken. Front. Physiol., 11, n°633. doi:10.3389/fphys.2020.00633
- Pullinger G.D., van Diemen P.M., Carnell S.C., Davies H., Lyte M., Stevens M.P., 2010. 6-hydroxydopamine-mediated release of norepinephrine increases faecal excretion of Salmonella enterica serovar Typhimurium in pigs. Vet. Res.,41, 68. doi:10.1051/vetres/2010040
- Råberg L., Sim D., Read A.F., 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Sci., 318, 812-814. doi:10.1126/science.1148526
- Rabot S., 2015. Axe intestin cerveau : comment le microbiote intestinal influence la répons eau stress. Bull. Acad. Vét. France.168, 267-273. doi:10.4267/2042/57938
- Rödel H.G., Bautista A., Hudson R., 2008. Why do heavy littermates grow better than lighter ones? A study in wild and domestic European rabbits. J. Phys. Behav., 95, 441-448. doi:10.1016/j.physbeh.2008.07.011
- Rodenburg T.B., van Krimpen M.M., de Jong I. C., de Haas E.N., Kops, M.S., Riedstra B.J., Nordquist R.E., Wagenaar J.P., Bestman M., Nicol C.J, 2013. The prevention and control of feather pecking in laying hens: identifying the underlying principles. Worlds Poultry Sci. J., 69, 361-373. doi:10.1017/S0043933913000354
- Rosell J.M., De la Fuente L., 2013. Assessing ulcerative pododermatitis of breeding rabbits. Animals, 3, 318-326. doi:10.3390/ani3020318
- Round J., Mazmanian S., 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol., 9, 313-323. doi:10.1038/nri2515
- Schneider D., Ayres J., 2008. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol., 8, 889-895. doi:10.1038/nri2432
- Schneitz C., 2005. Competitive exclusion in poultry––30 years of research. Food Control 16, 657-667. doi:10.1038/nri2432
- Shrestha M., Garreau H., Balmisse E., Bed’hom B., David I., Guitton E., Helloin E., Lenoir G., Maupin M., Robert R., Lantier F., Gunia M., 2020. Genetic parameters of resistance to pasteurellosis using novel response traits in rabbits. Gen. Sel. Evol., 52, 14p., doi:10.1186/s12711-020-00552-8
- Skuce P.J., Morgan E.R., van Dijk J. Mitchell M., 2013. Animal health aspects of adaptation to climate change: beating the heat and parasites in a warming Europe. Animal, 7, 333-345. doi:10.1017/S175173111300075X
- Suzuki K., 2018. The developing world of DOHaD. J. Dev. Orig. Health Dis., 9, 266-269. doi:10.1017/S2040174417000691Szendrö Z., Trocino A., Hoy S., Xiccato G., Villagrá A., Maertens L., 2019. A review of recent research outcomes on the housing of farmed domestic rabbits: reproducing does. World Rabbit Sci., 27, 1-14. doi:10.4995/wrs.2019.10599
- Tran T.S., Beaumont C., Salmon N., Fife M., Kaiser P., Le Bihan-Duval E., Vignal A., Velge P., Calenge F., 2012. A maximum likelihood QTL analysis reveals common genome regions controlling resistance to Salmonella colonization and carrier-state. BMC Genom., 13, 198. doi:10.1186/1471-2164-13-198
- Travel A., Petit A., Barat P., Collin A., Bourrier-Clairat C., Pertusa M., Skiba F., Crochet S., Cailleau-Audouin E., Chartrin P., Guillory V., Bellenot D., Guabiraba R., Guilloteau L., 2021. Methodologies to assess the bioactivity of a plant extract on redox balance, inflammation, health, welfare and performance in the chicken: the case of Melissa officinalis L. extract. Front. Vet. Sci., doi:10.3389/fvets.2021.759456
- Vaarst M., Alroe H.F., 2012. Concepts of animal health and welfare in organic livestock systems. J. Agric. Environ. Ethics 25, 333-347. doi:10.1007/s10806-011-9314-6
- Van de Ven L.J., van Wagenberg A.V., Debonne M., Decuypere E., Kemp B., van den Brand H., 2011. Hatching system and time effects on broiler physiology and posthatch growth. Poult Sci., 90,1267-75. doi:10.3382/ps.2010-00876
- Van der Waaij E.H., Van den Brand H., Van Arendonk J.A.M., Kemp B., 2011. Effect of match or mismatch of maternal-offspring nutritional environment on the development of offspring in broiler chickens. Animal, 5, 741-748. doi:10.1017/S1751731110002387
- Villemin M., 1981. La santé animale. Essai de définition tenant compte de la santé publique et de l’économie agricole. Bull. Acad. Vét. de France, 54, l385-392.
- Weström B., Arévalo Sureda E., Pierzynowska K., Pierzynowski S.G., Pérez-Cano F.J., 2020. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 11. doi:10.3389/fimmu.2020.01153
- Zepp M., Louton H., Erhard M., Schmidt P., Helmer F., Schwarzer A., 2018. The influence of stocking density and enrichment on the occurrence of feather pecking and aggressive pecking behavior in laying hen chicks. J. Vet. Behav.-Clin. Appl. Res., 24, 9-18. doi:10.1016/j.jveb.2017.12.005
- Zhai H.X., Liu, H., Wang S.K., Wu J.L., Kluenter A.M., 2018. Potential of essential oils for poultry and pigs. Anim. Nutr., 4, 179-186. doi:10.1016/j.aninu.2018.01.005
Abstract
Integrated animal health management can be defined as all the knowledge and practices mobilized by humans in a coordinated manner in order to build, preserve or recover the health of individuals or the herd within the breeding system. Its aim is to optimize animal health and the production cycle while reducing the use of antibiotics and antiparasitic agents that cause resistance problems in animals and humans. It is based on the joint mobilization of three complementary principles: (P1) preventing the onset of diseases by limiting risk situations and contact with harmful elements (pathogens, toxic elements), (P2) using resistant animals or developing their adaptive capacities, (P3) treating animals in a targeted manner (molecule, dose, duration). Health is built throughout the animal's life to ensure harmonious development and physical integrity of individuals. Numerous levers of action, grouped into six dimensions (1-living environment of the animals, 2-reproductive management, 3-herd management, 4-choices and practices with the animals, 5-feeding and 6-farming management) have been identified to achieve this objective. These levers can have a direct, delayed, or intergenerational effect on health. A coherent mobilization of many levers has allowed a significant reduction in the use of antibiotics in recent years, but there is still room for improvement in monogastric farming systems. In addition, the development of farming systems in line with societal demands (respect for animal welfare, short and local circuits, access to the outdoors) poses new challenges for integrated animal health management.
Attachments
No supporting information for this article##plugins.generic.statArticle.title##
 Views: 5793
Views: 5793
Downloads
 PDF: 147
PDF: 147
 XML: 50
XML: 50
Most read articles by the same author(s)
- Sylvie COMBES, Thierry GIDENNE, Samuel BOUCHER, Laurence FORTUN-LAMOTHE, Gérard BOLET, Gérard COUREAUD, What tools for more robust rabbits around weaning? , INRAE Productions Animales: Vol. 31 No. 2 (2018)
- Marie-Angélina MAGNE, Marie-Odile NOZIÈRES-PETIT, Sylvie COURNUT, Émilie OLLION, Laurence PUILLET, David RENAUDEAU, Laurence FORTUN-LAMOTHE, Managing animal diversity in livestock farming systems: which diversity? Which forms of management practices? For which benefits? , INRAE Productions Animales: Vol. 32 No. 2 (2019): Volume 32 Issue 2: Special Issue. Major challenges and solutions for livestock farming
- Alain DUCOS, Frédéric DOUHARD, Davi SAVIETTO, Marion SAUTIER, Valérie FILLON, Mélanie GUNIA, Rachel RUPP, Carole MORENO-ROMIEUX, Sandrine MIGNON-GRASTEAU, Hélène GILBERT, Laurence FORTUN-LAMOTHE, Contribution of animal genetics to the agroecological transition of livestock farming systems , INRAE Productions Animales: Vol. 34 No. 2 (2021)
- Hervé GARREAU, Mélanie GUNIA, The genomics of the rabbit: advances, applications and prospects , INRAE Productions Animales: Vol. 31 No. 1 (2018)
- Christelle KNUDSEN, Cécile BONNEFONT, Laurence FORTUN-LAMOTHE, Karine RICAUD, Xavier FERNANDEZ, Spontaneous liver steatosis in waterfowls: overview on current research and perspectives , INRAE Productions Animales: Vol. 31 No. 2 (2018)
- Thierry GIDENNE, Davi SAVIETTO, Laurence FORTUN-LAMOTHE, Yayu HUANG, Rabbit farming on pasture and under "Organic regulation" in France: systems operation, performances and regulation , INRAE Productions Animales: Vol. 35 No. 3 (2022)
- Joanna LITT, Christine LETERRIER, Laurence FORTUN-LAMOTHE, Rearing conditions of palmipeds and fatty liver production: from societal demands to a process of progress , INRAE Productions Animales: Vol. 33 No. 3 (2020)
- Nathalie LE FLOC’H, Anne BOUDON, Lucile MONTAGNE, Hélène GILBERT, Florence GONDRET, Bénédicte LEBRET, Louis LEFAUCHEUR, Isabelle LOUVEAU, Élodie MERLOT, Marie-Christine PÈRE, Marie-Christine MEUNIER-SALAÜN, Armelle PRUNIER, Hélène QUESNEL, Welfare and health of gestating sows and growing pigs , INRAE Productions Animales: Vol. 34 No. 3 (2021)
- Anne COLLIN, Vincent COUSTHAM, Jacob Kokou TONA, Sophie TESSERAUD, Sandrine MIGNON-GRASTEAU, Bertrand MÉDA, Anaïs VITORINO CARVALHO, Yann GUYOT, Sandrine LAGARRIGUE, Frédérique PITEL, Tatiana ZERJAL, What mitigation and adaptation strategies for poultry production in the face of climate change? , INRAE Productions Animales: Vol. 37 No. 1 (2024)
- Vincent COUSTHAM, Charlotte, Chloé, Anne, Ingrid, Julie DEMARS, Guillaume DEVAILLY, Mireille MORISSON, Marianne HOUSSIER, Sandrine LAGARRIGUE, Sonia MÉTAYER-COUSTARD, Sandrine MIGNON-GRASTEAU, Stéphane PANSERAT, Angélique PETIT, Anaïs VITORINO CARVALHO, Tatiana ZERJAL, Frédérique PITEL, Epigenetics, genes and the environment: what importance for breeding practices and selection methods in poultry? , INRAE Productions Animales: Vol. 36 No. 4 (2023)

