New early nutritional strategies to the benefit of poultry production (Full text available in English)
Header
Growth, development and robustness: should we wait for the outbreak to happen before reacting? Enriching the egg with certain nutrients of interest is a new production strategy that aims to improve the quality of the chick at hatching, increase the efficiency of its metabolism and obtain more robust adult animals that can adapt to given rearing conditions, while also controlling the development of quality products.
Introduction
The world's population continues to grow. The need for both cereals and meat is increasing considerably. Poultry is a source of animal protein that is popular with humans, quick to produce, cheap and not affected by religious prohibitions. Poultry production is steadily increasing (+2% per year with 107 MT in 2013) and is second only to pork (114 MT) and far ahead of beef (68 MT) (Box 1).
Box 1. Evolution of world meat production (in Million Tons) (Source: Annual report "OECD and FAO Agricultural Outlook", 2014).
In 2014, world poultry meat production was estimated at 110.5 MT, an increase of more than 3% compared to 2013 (107 MT). FAO's agricultural outlook shows that poultry meat production can be expected to increase by 1.8% per year from 2015 to 2024. By 2020, the poultry industry would then become the world's leading meat producer.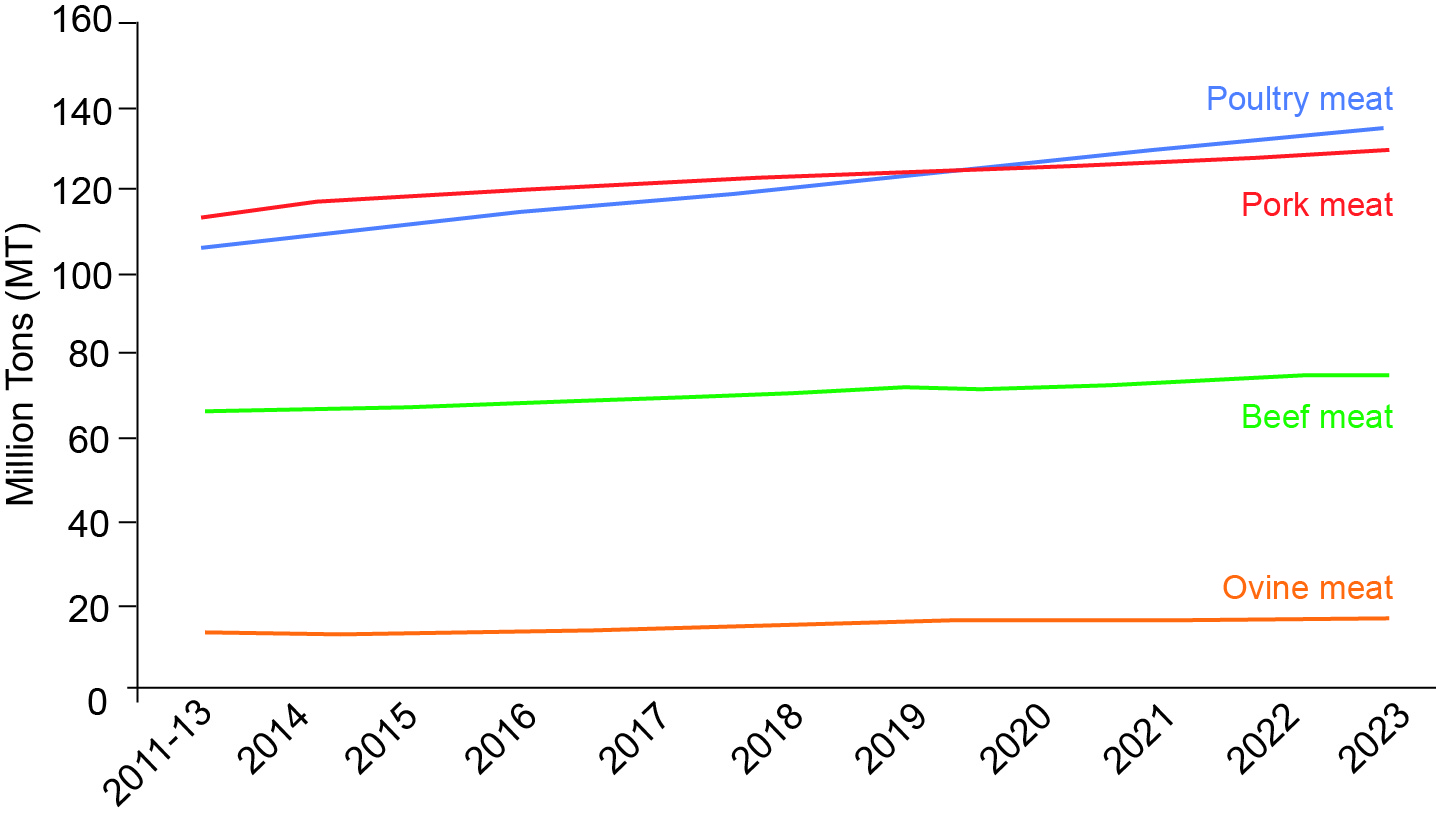
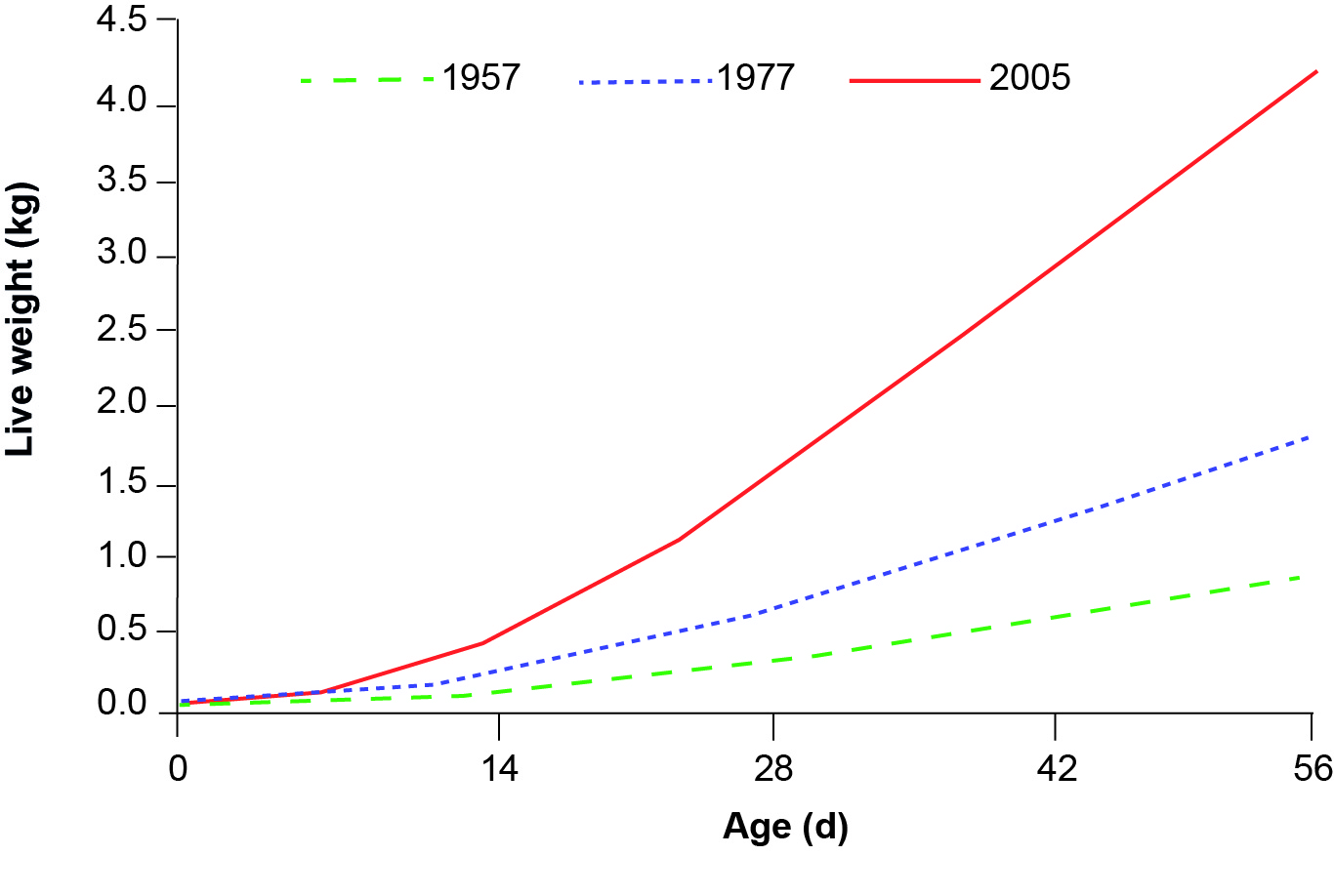
To meet this growing demand, broilers have mainly been selected for their rapid growth rate. The increase in body weight of broilers per year is about 3.3% (Zuidhof et al., 2014) (Figure 1). As the chicken is slaughtered at a target live weight of 1.7_3.5 kg, the increase in growth rates have resulted in a decrease in slaughter age of about 1 day per year. In Europe, the rearing period is less than 6 weeks for "standard" broilers (Arnould et al., 2011). The skeletal, immune and cardiovascular systems have not kept pace with the massive increase in muscle mass (Havenstein et al., 2003; Tona et al., 2003; Hocking, 2010).
This selection strategy has inevitably been accompanied by undesirable effects such as a higher frequency of ascites, the appearance of skeletal and meat defects, some immunosuppression in animals, an increase in their susceptibility to infectious diseases and an increase in the incidence of metabolic diseases (Emmerson et al., 1997; Decuypere et al., 2003; Havenstein et al., 2003). It should be noted that feed requirements have been established essentially on the basis of the growth performance of young animals without taking into account other functions such as immune function or, more broadly, the "health" valence. For breeding stock, ad libitum feeding leads to excessive obesity with very low reproductive capacity and high morbidity and mortality. This practice also has a serious impact on bird welfare (Decuypere et al., 2006, 2010; De Jong and Guémené, 2011) and on animal integrity, which goes beyond health and welfare concerns (Decuypere et al., 2010). The integration of the two aspects of growth and reproduction is relatively incompatible and is recognised as the "rapid growth versus reproduction - health" paradox.
Figure 1. Changes in live weights of unselected broilers from 1957 and 1977, and Ross 308 chicken (2005) (Adapted from Zuidhof et al. 2014).
In the "broiler" sector, a restriction on the diet of breeding stock is therefore carried out in order to maximise egg and chick production. Nowadays, female breeders receive on average 25% of what they would normally consume in ad libitum mode. This severe dietary restriction allows the body weight trajectory to be kept within well-defined limits to ensure proper reproductive performance in adulthood. However, it has a negative impact on the offspring. In fact, restriction of broodstock, sustained egg production levels or other stresses can cause egg composition to vary, which can then lead to eggs that are deficient in the critical nutrients needed for proper chick start-up. Under these conditions, and with a delay between hatching and rearing that can be as long as 48_72 hours, as well as transport conditions that are often sub-optimal, the starting period (1st week after hatching) is then delicate, and performance is significantly impacted.
In growing animals, any quantitative (energy and protein levels) or qualitative (type of diet, nutrient intake or distribution pattern) changes in the diet affect metabolism. This has consequences on energy consumption, feed efficiency, nutrient distribution between tissues or organs and thus on body composition and meat quality (Tesseraud et al., 2014). This also applies to a developing embryo. Nutrient supplementation of the egg via maternal feeding or via in ovo feeding are then innovative strategies to optimise the supply of nutrients to the embryo and improve the starting conditions of the chicks and their robustness. The incubation period and the first week of age are essential for their health, well-being and growth performance (Bigot et al., 2003; Yassin et al., 2009). The aim of such approaches is to optimise the nutritional intake of the breeding females, developing embryos and/or starter chicks in order to obtain chicks of better quality in terms of robustness, growth and/or body composition by taking advantage of the embryonic plasticity of nutrient utilisation.
1. Sources and nature of nutrients and active molecules available to the developing embryo
The duration of the embryonic development of the bird varies according to that of the life of the bird because both correspond to its growth rate. It varies from 10 days in the Cowbird (Molothrus sp.) to 80 days in the Royal Albatross. Ducks require 28_36 days (for the Beechnut and Barbary Albatross respectively), geese require 30_35 days and domestic chickens require 21 days. When not specified, the species being discussed in the following will be chicken. As the slaughter weight of animals is reached earlier and earlier (between 35_42 days), embryonic development accounts for 33_38% of the life of a modern broiler. When the incubation period and the perinatal period (up to 4 days after hatching) are included, together they account for more than 50% of the animal's life (Druyan, 2010). In oviparous vertebrates (such as birds), embryonic development takes place entirely in the egg, independent of the mother. The egg then forms a natural closed enclosure that contains all the elements necessary for the survival, development and protection of an embryo when incubated under the right conditions (temperature, humidity and inversion) (Box 2). The embryo uses the nutrients of the egg without the possibility of waste disposal, since only gas exchange takes place with the outside environment.
Box 2. The hatching egg and embryonic appendages.
Physical defence of the embryo is primarily ensured by the shell, which constitutes a protective envelope. Formed of calcium carbonate crystals, the shell is porous. It thus allows gas exchange but induces water losses. Three annexes allow the embryo to develop independently of the mother. The amnion delimits the cavity in which the embryo is bathed. It isolates the embryo and protects it. The yolk sac, whose wall is highly vascularized, contains the yolk reserves (yolk or yellow). The allantoic sac serves as a reservoir for the waste products, particularly nitrogenous (uric acid), eliminated by the embryo. Its vascularized wall (chorioallantoic membrane) is the site of respiratory exchanges (via the pores of the shell). In addition, minerals from the shell can be absorbed from this allantoic sac and transferred to the embryo for the calcification of its skeleton. It is also a storage organ for many free amino acids and related compounds. These compounds are important for the nutrition of the embryo during the late incubation phase (diagram adapted from Da Silva, 2017).
The main source of nutrients for the embryo during the first two weeks of its development is the yolk, which is composed mainly of lipids and proteins. The carbohydrate content of the egg is very limited; its concentration is less than 1% of the total nutrients and the percentage of free glucose is only about 0.3% (Campos et al., 2011). In the yolk sac, with the exception of immunoglobulins, most of the proteins, triglycerides, phospholipids and cholesterol are synthesised by the liver of the breeding hen. Lipids and proteins account for 62.5% and 33% of the dry matter of the yolk, respectively (Powrie et al., 1986). Lipids are the main source of energy for the developing embryo (Moran, 2007; Cherian, 2015). All the proteins in the egg yolk also constitute a reserve of nutrients. In addition to their nutritional value, many proteins also have their own biological activities, such as transport and storage of vitamins ("riboflavin-binding protein", "vitamin-D-binding protein", avidin) or metal ions (ovotransferrin, phosvitin), lipase-inhibiting activities (apovitellenin), etc. (Moran, 2007; Bourin, 2011; Cherian, 2015). Finally, yolk also contains fat-soluble molecules such as vitamins A, D, E and carotenoids. The latter come exclusively from the mother's diet. Molecules with antimicrobial potential are also present in egg yolk (Bourin et al., 2011).
From 14 days of development, the embryo will be surrounded by a double envelope consisting of the amnion and the allantoid. In the final third of development for the hen, the embryo will therefore have access to other nutrients present in these two appendages (rich in water and protein). After 20 days, the chick is in its hatching position, the beak has pierced the air chamber and lung breathing has begun. After 21 days of incubation, the chick finally comes out of its shell. The allantoid, which served as its lungs, dries out because the chick uses its own lungs. During hatching, the amnion and allantoid are eliminated with the shell, the albumen has been used and the remaining yolk retracts into the abdomen of the animal. The chick then switches from a mainly lipid and protein diet to a carbohydrate diet as soon as it is transported and put in place at the breeder's with access to a cereal-rich feed.
2. Changes in the environment of the embryo via maternal feeding and consequences on the development of offspring phenotypes
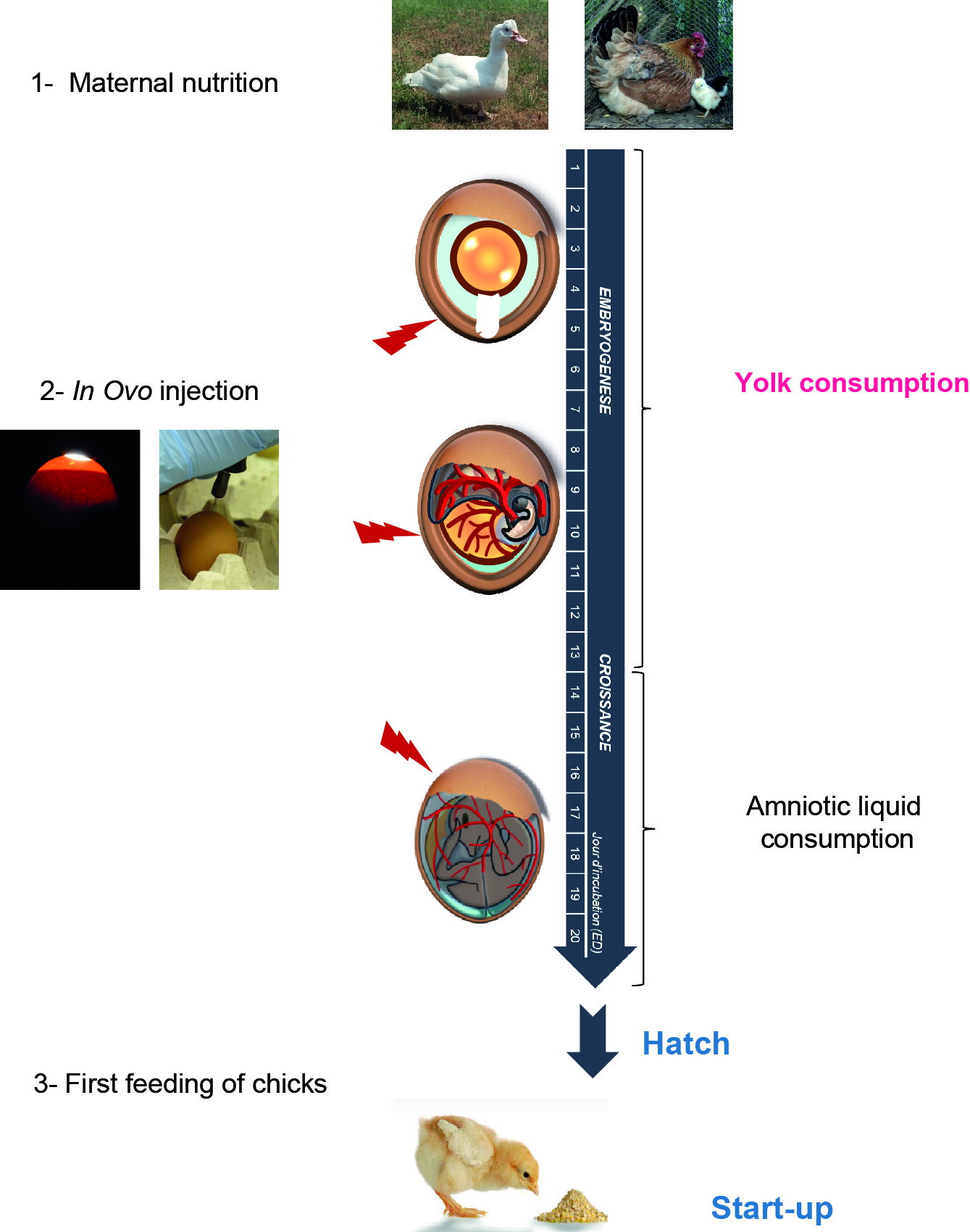
The development of the embryo depends on the environment in which it is immersed and the nutrients available. One way of modulating egg composition is by altering the mother's diet (Kidd et al., 2005; Rao et al., 2009) (Figure 2). This environment can be modulated to influence the physiological and morphological development of embryos, with consequent effects on the development of the chick phenotype (Ho et al., 2011). Links between parental nutrition, egg composition and the subsequent behaviour of the animals (Aigueperse et al., 2013), their performance (Bergoug et al., 2013), and their susceptibility to disease have been established. The mechanisms underlying these maternal effects are not always fully deciphered or understood.
Figure 2. Early nutritional events that may modulate metabolic programming and animal phenotype (Adapted from Da Silva, 2017 and Métayer-Coustard et al., 2017).
2.1. Changes in the phenotype of chicks as a result of rearing practices experienced by their mothers
In addition to the severity of the dietary restriction applied to the breeding females, the timing and duration of dietary restriction during the rearing period is a determining factor in subsequent egg-laying performance and the development of the phenotype of the offspring. For example, feed restriction before the onset of sexual maturity can affect a range of circulating hormones such as thyroid or somatotropic hormones (Bruggeman et al., 1999). These hormones influence the subsequent performance of offspring.
Protein deficiency or fasting also alters the endocrine status of animals (Scanes and Griminger, 1990). Hormonal changes in the egg have been demonstrated in low-protein diet distribution programs (Rao et al., 2009). This diet not only altered both the egg laying rate and egg weight but also the amount of leptin in the yolk and the expression of a number of genes expressed in the yolk sac, hypothalamus or muscle of the offspring. The chicks had lower hatching weights but faster post-hatching growth. More recently, it has been shown that protein diets administered to females negatively impacted reproductive performance but improved offspring performance (Lesuisse et al., 2017). The authors were also able to demonstrate multigenerational effects of this type of diet on offspring performance (Lesuisse et al., 2018a and 2018b; Li et al., 2018).
Maternal effects have also been described on the development and body composition of chicks from restricted dams. These offspring, fed ad libitum, showed less growth and greater adiposity compared to animals with less restricted dams (van der Waaij et al., 2011). This management resulted in significant economic losses due to reduced body mass and feed efficiency. Spratt and Leeson (1987) also showed differences in protein deposition and carcass adiposity in animals from breeding hens fed diets with different energy and protein content.
In addition to their nutritional value, the diets of female reproducers may have an olfactory signature that the embryos are able to pick up. The odours present during incubation, from the early stages of development of the olfactory system when the embryos have not yet adopted air breathing, may then influence the future food preferences of the young (Bertin et al., 2012; Aigueperse et al., 2013).
2.2 Changes in the composition of the egg and the environment of the embryo through maternal feeding
The composition of the egg is fairly stable and the hen's diet does not affect the composition of the egg's major constituents (lipids or proteins). However, the levels of essential nutrients such as fatty acids, vitamins, trace elements, carotenoids or certain amino acids can be modulated in the egg via the mother's diet and induce changes in the performance of the offspring (for reviews, see Kidd, 2003; Calini and Sirri, 2007; Rühl, 2007). Some examples, which are not exhaustive, are detailed below.
a. Fats and polyunsaturated fatty acids
Lipids are the major nutrients in the yolk that are available to the developing embryo. The oxidation of fatty acids (FA) covers practically all the energy demands of the embryo. Fatty acids are therefore essential for embryonic development, bird growth, the development of the central nervous system and the immune system (Noble et al., 1984; Ding and Lilburn, 1996; Cherian, 2015). In hens, Menge et al. (1974) showed that depletions of essential fatty acids caused later hatching and that offspring showed slower growth compared to a control group.
The structure of lipoproteins is stable but the balance of fatty acids can be modulated via the mother's diet. Polyunsaturated fatty acids (PUFA) will vary according to the lipid source added to the food. The n-6 fatty acids (FA ω6), linoleic (C18:2) or arachidonic (C20:4), are mainly found in soybean, sunflower or safflower oil. The linoleic acid content can thus be multiplied by a factor of 2_2.5. The n-3 fatty acids (FA ω3), linolenic C18:3, eicosapentaenoic (EPA) C20:5, docosahexaenoic (DHA) C22:6, are found in fish, flax, millet, rapeseed oils or in microalgae. The levels of the total ω3 can be multiplied by 6_7 times in the egg. Linolenic acid can be increased by a factor of 25, especially with linseed oil and DHA can be increased by a factor of 10 with fish oil. Variations in fatty acid composition and balance in the yolk can not only affect the hatchability, growth and performance of the offspring (Koppenol et al., 2015) but also their passive immunity (Wang et al., 2004). Several studies have shown that a maternal diet rich in omega 3 has olfactory properties that are transmitted to the egg (Leeson et al., 1998; Gonzales-Esquerra and Leeson, 2000) and are perceived by the embryos. These odours represent an interesting tool to reduce food neophobia in offspring (Aigueperse et al., 2013).
Numerous studies in humans and mouse models have shown a positive effect of long-chain FA ω3 (FA ω3 LC) on brain development and function. Ducks can express deleterious behaviours such as nervousness and pecking when reared. Enrichment of eggs and thus embryos with FA ω3 LC can be achieved by feeding ducks a diet containing DHA and linolenic acid (microalgae and linseed oil) (Baéza et al., 2017). This diet does not affect their egg-laying and reproductive performance and has no effect on the weight and lipid content of egg yolks. On the other hand, yolk lipids are enriched with FA ω3 and ducklings from ducks fed an FA ω3-enriched diet have a live weight greater at hatch (D0), D28 and D56 and a lower feed conversion ratio for the growing period. Enrichment of the duck feed with FA ω3 LC also reduces the frequency and severity of pecking in ducklings and reduces the duration of tonic immobility tested at 19 days of age. Reduced hyperactivity and reduced stress responsiveness in ducklings from these ducks was also observed.
b. Vitamins and minerals
Mineral and vitamin supplementation via the mother's diet has often been studied to solve skeletal mineralisation and leg problems. Avitaminoses in birds mainly result in a decrease in egg laying and a drop in hatching rate (Adrian, 1958). Vitamin A deficiency doubles the frequency of embryo malpositioning in the egg (Polk and Sipe, 1940). Vitamin E deficiency will result primarily in disintegration of the blood vessels of the blastoderm (Adamstone, 1931; Adamstone, 1941). Around the fourth day of development, a ring may also form in the blastoderm which interrupts or inhibits yolk circulation and causes the death of the embryo. A reduction in body, leg and wing length may also be noted (Ferguson et al., 1954). Vitamin D3 regulates calcium flow through the chorioallantoic membrane (CAM) in a unidirectional and active process. A vitamin D-deficient diet leads to a decrease in Ca++ transport across the CAM and a decrease in Ca++ accumulation in the embryo (rickets in the young, bone deformation), as well as an increase in late embryonic mortality (poor position, beak unable to pierce the shell).
Fat-soluble vitamins (vitamin A, E or D) as well as water-soluble vitamins (riboflavin, B12, thiamine, biotin, folic acid, etc...) can be enriched in the egg via the females' diet. Vitamins are provided in the form of supplements in the feed according to calculated needs, which are dated and no longer necessarily correspond to the needs of today's animals. Vitamin A is produced by the hen from carotenoids found in the feed. Carotenoid contents will vary according to the plant sources used. Their yield of deposition in the egg yolk is variable from one source to another. These carotenoids will give the egg yolk its colour, which depends on the quantity of carotenoids ingested, their colouring capacity and their stability. They will also have antioxidant properties. Vitamin D is only effective if the diet contains sufficient calcium and phosphorus. Unlike vitamin D3, vitamin D2 is not very useful for the bird (effectiveness of less than 10% compared to vitamin D3). Vitamin E, essential for fertility and brain development, is only effective if selenium is present in sufficient quantities. Minerals such as iodine, selenium, magnesium, zinc, copper or manganese can also be fortified in eggs (Surai and Parks, 2001; Jiakui and Xialong, 2004; Chinrasri et al. 2013; Favero et al. 2013; Saunders-Blades and Korver, 2015; Torres and Korver, 2018). Selenium can be increased by a factor of 5_10 and its content will be higher in egg white. This element is essential for antioxidant control in birds.
c. Amino acids
Amino acid requirements of female breeders had historically been defined by measuring the response of animals in terms of egg production. With increasing evidence of the effects of amino acids on offspring phenotype, these requirements are now being re-evaluated. Studies testing different levels of digestible lysine (Ciacciariello and Tyler, 2013) or arginine (Muller Fernandes et al., 2014) in maternal feed have thus shown positive effects of supplementation on offspring (e.g. optimisation of performance, carcass yield, abdominal fat content and bone quality for arginine supplementation). The mechanisms involved are still little studied but in the case of methionine, an essential amino acid for the bird, it was concluded that the metabolism of the offspring was altered in response to the diets of the breeders. Indeed, Brun et al. (2013 and 2015) showed that a diet restricted specifically to methionine distributed to ducks modified the hepatic metabolism of their offspring and could therefore induce different phenotypes in ducklings. A significant result of this study was the significant interaction between maternal diet and sex on the foie gras weight of the offspring. The 20% increase in male foie gras weight in this study could be used to reduce feeding time and associated costs, thus partly meeting a societal expectation to improve the welfare of farmed animals.
3. Egg supplementation by in ovo feeding
3.1 General information on in ovo nutrition
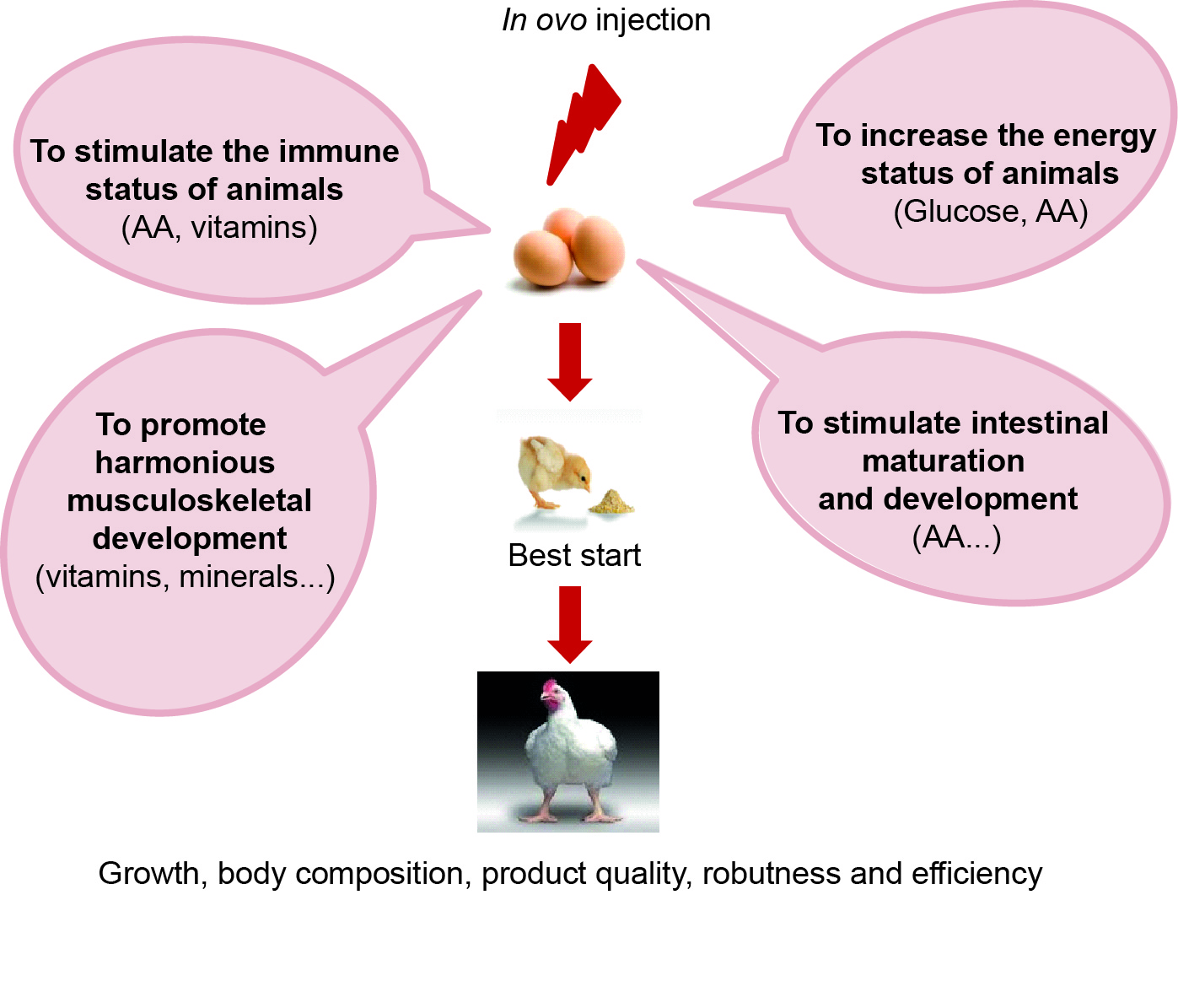
Considering that the formation and development of tissues (such as muscle) take place during the 21 days of embryonic development, which accounts for 33_38% of an animal's total life, it is likely that a physiological or metabolic disorder during this early development in the bird could significantly alter post-hatching weight gain and carcass characteristics (Velleman, 2007; Grodzik et al., 2013). The rapid development and growth of currently selected bird strains may result in insufficient nutrients in the egg for optimal tissue development (Grodzik et al., 2013). It is therefore necessary to act as early as possible on the metabolic programming of embryos in order to optimise their subsequent performance. Innovative nutritional strategies such as in ovo feeding by injecting nutrients directly into the egg have recently been developed to ensure a supply of readily available nutrients to support and accelerate the maturation of the digestive tract, optimise chick development and growth and thus obtain better quality chicks (Kadam et al., 2013; Roto et al., 2016; Gao et al., 2017; Ghanaatparast-Rashti et al., 2018; Peebles, 2018) (figure 3). Supplementation of the egg with different types of compounds is therefore aimed at providing additional nutrients to enable the chicks to cope with post-hatching delays of up to 48_72h and to modulate major functions. These nutritional strategies thus aim, for example, to respond to new challenges in the sector for which the use of antibiotics is strongly denounced and new practices aim to reduce or even eliminate their use in farms, which leads to production and profits losses for the breeders (Castanon, 2007).
Figure 3. In ovo injections: which ones and for what effects?
The hatching percentage and the weight of the chicks depend on the substance injected, the site and time of injection of the nutrients into the egg. In early development, compounds are injected as close as possible to the germinal disc (Ebrahimi et al., 2012). Later, the nutrients are administered into the yolk sac, which has a large surface area capable of absorbing nutrients. However, it appears that even with completely safe components, hatching is affected when injections are given early in the first two weeks of embryonic development in hens. After 17 days of incubation, when the yolk sac is resorbed, injections are made into the inner tube or amnion. These later injections often have less adverse impact on hatchability and embryonic mortality. The amnion is the preferred site since at the end of incubation the embryos ingest the liquid present there. These injections are often carried out at the time of egg transfer from the incubator to the hatching pen at the same time as in ovo vaccinations (around 18 days of incubation). In ovo vaccinations, carried out at the end of the incubation period, make it possible to vaccinate animals early without affecting their survival or hatchability (Breedlove et al., 2011). Handling of hatching animals is thus limited and the stress generated in the post-natal period is minimised. These practices are faster and allow for greater uniformity in the delivery of the vaccine dose or nutrient solution. Nutrient solutions injected in practice are often complex.
Because the bird is oviparous, it is an ideal model for distinguishing direct nutrient effects from maternal effects. Indeed, maternal nutrition can influence both the environment in which the embryo develops (the main source of nutrients for the bird) but also the way in which genes regulating metabolism are expressed via the transmission of maternal epigenetic markers (Frésard et al., 2013). This avian model thus makes it possible to understand and control both the direct (via in ovo injections) and indirect (via the mother) effects of nutrition on the regulation of metabolism and its long-term effects on growth, body composition and development of the offspring's phenotype.
Recent work has shown that it is thus possible to modify animal metabolism by exogenous inputs of various substances directly into the egg, including amino acids, energy substrates, electrolyte solutions, hormones, nucleotides and vitamins at key moments of embryogenesis by in ovo injections (Gore and Qureshi, 1997; Henry and Burke, 1999; Ohta et al., 1999; Kocamis et al., 1999 and 2000; Tako et al., 2004; Uni et al., 2005; Kadam et al., 2008; Zhai et al., 2011c; Mc Gruder et al., 2011; Kornasio et al., 2011; Bakyaraj et al., 2012; Li et al., 2016; Neves et al., 2017; Gao et al., 2017). These practices induce differences in chick performance at hatching (weight, glycogen stock, net yield), which can be maintained until slaughter age. However, the mechanisms of early guidance of metabolism at these periods remain largely unknown.
3.2 Enrichment of eggs with energy substrates
Hatching is a critical milestone in a bird's life. This energy-intensive process affects hatching, morbidity and mortality rates and, in the longer term, impacts on health, growth and the quality of the final product. At hatching, embryos preferentially use glucose rather than fatty acids for energy production. In fact, the quantity of oxygen in the egg is limited, and for the same quantity of oxygen consumed, the oxidation of glucose allows more energy to be obtained than with lipid catabolism. Because of the limited carbohydrate content in the egg, less than 1% of the total available nutrients and only 0.3% of free glucose, the maintenance of glucose homeostasis during late embryonic development depends on the amount of glucose stored as glycogen in the liver and glucose generated by gluconeogenesis from proteins (especially muscle) (Campos et al., 2011). Glycerol from hepatic triglyceride metabolism (from egg yolk) is a major and indispensable substrate for glycogen synthesis in liver and muscle at the end of incubation (Sunny and Bequette, 2011). Between 15 and 19 days of incubation, the liver is the site of an active metabolism, leading to a transfer of glucose and fatty acids to the cervical muscle ("pipping muscle": muscle used when digging the shell to break it), which is, gradually enriched in glucose, glycogen and protein for hatching (Pulikanti et al., 2010). Low liver glycogen levels are associated with later and longer hatching times and reduced hatching weight of chicks. The embryo must mobilise more muscle protein to provide gluconeogenesis-oriented amino acids, limiting growth in late hatching and during the first week of start-up (Pearce, 1971). The use of protein as an energy source will be to the detriment of muscle development. At the end of embryogenesis, pectoral muscle yield decreases significantly (Guernec et al., 2003). An overexpression of atrophy-related genes such as atrogin-1 and MuRF1 observed at hatching compared to day 18 of embryogenesis could partially explain this pectoral muscle melting (Everaert et al., 2013). To limit the use of fatty acids and the proteolysis of muscle proteins for energy purposes, injections of carbohydrates alone or combined with other nutrients of interest have been performed in ovo (Retes et al., 2018).
In ovo supplementation has often been aimed at increasing the amount of sugars available in the egg and thus sparing the use of amino acids for gluconeogenesis (Kornasio et al., 2011). In a recent study, Retes et al. (2018) were able to demonstrate that the results were dependent on the type of sugar injected, the injection site (yolk, albumen, amnion, allantoic fluid or air chamber), the development stage of the embryo and the genetics of interest in the studies. Uni et al. (2005), for example, showed that administration at 17.5 days of a solution containing carbohydrates (maltose, sucrose and dextrin) and a metabolite of leucine (β-hydroxymethylbutyrate) increased the hatching weight of chicks by 5_6%, liver glycogen stores by 2_5 times and pectoral muscle yield by 6_8% compared to control chicks whose eggs were not supplemented. These improved performances were still measurable at 25 days of age. Supplying sugars as an energy source by injection into the amnion at the end of incubation (17.5 d) may also accelerate intestinal development by increasing the size of villi and thus increasing the capacity of the gut to digest disaccharides (Tako et al., 2004). This supplementation may have trophic effects on the small intestine and improve the development of caliciform and mucin-secreting cells (Smirnov et al., 2006), which certainly explains the higher weights of animals from supplemented eggs. Other sugars, such as fructose, are not recommended as several studies show a decrease in hatchability of fructose-supplemented batches as well as lower animal weights (Zhai et al., 2011).
Other nutritional supplements, such as amino acids (Li et al., 2016), glycerol (Neves et al., 2017), L-carnitine and creatine pyruvate (Zhao et al., 2017) have been injected into different egg compartments at different stages of development in order to modify embryo metabolism and the phenotype of the hatching animals (quality and weight of the chick, body composition, quantity of liver glycogen, muscle yield etc.). The animals are sometimes kept beyond the hatching stage to check whether the effects observed at hatching remain perennial. With creatine pyruvate supplementation at 12 mg per egg, for example, the energy status of the animals was altered. This supplementation led to differences in the weights of the animals and Pectoralis major that persisted up to 21 days of age (Zhao et al., 2017).
3.3 Enrichment of eggs with amino acids
Embryonated eggs contain all the amino acids necessary for the growth and development of an embryo. As reported by Kucharska-Gaca et al. (2017), the amino acid composition of an egg has changed very little over the years while the nutrient requirements of birds during embryonic development have changed. Amino acid administration in ovo can provide poultry firms with an alternative method to improve hatching weights (Ohta et al., 2001). This difference in hatching weight persists up to 56 days of age in some studies (Al-Murrani, 1982). Administration of amino acids may also induce an increase in lymphoid organ mass. Other strategies consist of administering a single amino acid, such as threonine (Kadam et al., 2008) or DL-methionine (Coskun et al., 2014). Some injected amino acids, such as arginine or glutamine, are used for the synthesis of other amino acids. These supplements, in addition to their positive effects on animal weight, decrease hatching time (Shafey et al., 2014). The contribution of amino acids alone or in association with others could stimulate protein synthesis and muscle protein accretion, but the mechanisms involved remain to be understood. Arginine, considered an essential amino acid in birds, is involved in protein synthesis. It can also be converted to glucose and used in many metabolic pathways that produce active compounds that help maximise the potential development of an embryo by stimulating the secretion of growth hormone (Tong and Barbul, 2004). The effects of arginine have been confirmed in other birds such as quails and turkeys (Foye et al., 2007) with other effects demonstrated such as an increase in the number of hatched chicks, their weight at 7 and 42 days of age, their weight gain and feed efficiency in quails and an increase in digestive enzyme activity (sucrose, maltose and leucine amino-peptidase activities) in the digestive tracts of turkeys.
3.4. Enrichment of eggs with vitamins and trace elements
The efficacy of vitamins C, E, D3 and B9 (folic acid) on embryonic health and development has been reported by several teams in the literature (Peebles, 2018). As shown previously, the embryo draws its energy largely from the yolk. This use is the result of oxidative processes that produce free radicals, leading, among other things, to the degradation of polyunsaturated fatty acids in cell membranes. Vitamins, such as vitamin E or vitamin C, limit the negative effects of free radicals and protect the embryo (Surai et al., 2016; Peebles, 2018; Araújo et al., 2018). Depending on doses, ages and injection sites, the reported results may be different (increased antioxidant capacity, better immunity, positive effect on growth, etc.).
Administration of vitamins A, B1, B2, B6 or Eat 14 days of embryonic development may contribute positively to animal growth (vitamins B1 and B2) and/or modulate animal immunity and robustness (Goel et al., 2013). The relative weight of Fabricius bursae is thus higher in 42-day-old animals that received vitamins B1, B2 and E in ovo and thymus weight is higher in the groups that received vitamins A, B6 and E. The group having received vitamin B1 shows a better humoral response. Vitamin E, on the other hand, will not increase the performance of the animals but will improve hatchability and stimulate the post-hatching immune status of the chicks (Salary et al., 2014). Vitamin E injection will increase the resistance of chicks to diseases such as avian influenza or infectious bronchitis. In this experimental group, higher levels of IgG, IgM and IgA could be demonstrated.
To stimulate the growth and development of bone or its mineralisation, in ovo injections of vitamin D have been practiced. Vitamin D is involved in calcium metabolism, and previous research has shown better bone resistance (mineralisation) with higher levels of vitamin D in the diet. However, in ovo injection of 25-hydroxycholecalciferol on day 18 of embryogenesis in anticipation of higher bone mineralisation at hatching and 21 days of life does not always affect bone quality in hatching animals, perhaps because the chick could not find the extra calcium and minerals due to the constrained composition of the egg or the injection was made too late in the development of the chick (Bello et al., 2014). The combination of vitamin D3 and minerals injected earlier (E17) appears to have positive effects on the bone properties of hatched chicks and older chickens (Yair et al., 2015). While some vitamins may appear to be ineffective in some forms, others may be toxic. For example, vitamin D3 may induce calcification of soft bones, compromising hatching and increasing embryonic death. Supplemented in the form of 25-hydroxycholecalciferol, which is biologically more active than vitamin D3 and more stable and less toxic than 1,25-dihydrocholecalciferol (Soares et al., 1995), it has no deleterious effect on embryogenesis, skeletal development or the hatchability of chicks (Bello et al., 2013).
Egg supplementation with selenium, an essential element in antioxidant control in birds, has been tested on the effectiveness of protecting chicks against necrotic enteritis (Lee et al., 2014). An overall protective effect in chickens against experimental necrotic enteritis was demonstrated in this study. Selenium is thought to have this effect by controlling the activation of neutrophils, macrophages and B lymphocytes.
4. How nutrients can contribute to the metabolic programming of animals and the development of their phenotype.
Determining the mechanisms by which egg nutrients regulate cell metabolism, signalling, gene expression and function is essential to improve nutrient utilisation, poultry production efficiency and animal robustness. Few mechanisms have been deciphered to date in birds. The studies, which are not exhaustive and referenced, often do not address the study of the mechanisms involved in the metabolic reprogramming of embryos. These could be metabolic regulations similar to those described in later breeding periods. The importance of certain amino acids (lysine and methionine) for the regulation of protein and energy metabolism in growing animals is well established, with marked effects on chicken growth and product quality (Berri et al., 2008; Métayer et al., 2008; Tesseraud et al., 2009). Such regulation by amino acids used as substrates or as signal nutrients allows modulation of protein-energy metabolism (Tesseraud et al., 2003; Métayer-Coustard et al., 2010). In addition, epigenetic-type modifications, i.e., transmissible and reversible changes in gene expression, without altering the DNA sequence, could also be involved, particularly when molecules donating methyl groups such as methionine or other micronutrients are injected (Anderson et al., 2012; Donohoe and Bultman, 2012; Veron et al., 2018).
In mammals, more data are available. Maternal malnutrition leads to low birth weight of the offspring. Subsequent food abundance in the environment leads to compensatory growth (Fagerberg et al., 2004). Maternal nutrition during gestation alters gene expression in offspring through epigenetic alterations such as DNA and histone modifications (Simmons, 2011). The epigenome is particularly dynamic during embryogenesis and the DNA methylation necessary for normal tissue development is established during early development. DNA methylation is an important epigenetic factor for the maintenance of genetic silence based on the provision of methyl groups. Histones are also influenced by numerous post-translational modifications (phosphorylation, ubiquitination, ADP-ribosylation, sumoylation and glycosylation). Substrates and co-factors involved in the supply of methyl groups are considered particularly important during early stages of development (Sinclair et al., 2007b). Thus, manipulation of the sulphur amino acid content of early diets can induce changes in cell function that have implications for the animal's development, long-term growth and health. Sulphur amino acids (Met) as well as other amino acids (Gly, His and Ser), vitamins (B6, B12 and folate) or micronutrients (betaine and choline) play a key role in providing methyl donors for DNA and histone modifications (Anderson et al., 2012).
In various species, suboptimal diets of folic acid, choline, methionine and/or vitamin B12, and those deficient in methyl groups distributed around the conception, program the insulin axis and increase the carbohydrate metabolism of the offspring. Altmann et al. (2012 and 2013) showed that both restricted and excess protein during gestation in pigs could alter the expression of key genes involved in methionine metabolism in the liver and muscle of the offspring. These genes are important for chromosome condensation and overall DNA methylation. In rats, a diet deficient in methyl groups alters the liver proteome of the offspring in adulthood (Maloney et al., 2013). For these reasons, nutritional supplementation, particularly of micronutrients, is being developed in birds in an attempt to programme the animals' metabolism at an early stage and thus enable them to make better or different use of the nutrients available to them.
Conclusion
The very early manipulation of the environment in which the embryo develops is very promising for analysing the influence of early conditions on the development of long-term phenotypes, whether on growth criteria, body composition, tissue characteristics, robustness to challenges or adaptation to different breeding conditions. These approaches aim to increase the efficiency and quality of production (chick and meat) as well as the adaptability of animals to different environments. In addition to the developmental, health and welfare benefits for the animals, these feeding strategies can also address environmental and economic interests.
Trans-generational transmission studies are still anecdotal in the literature in birds and have yet to be developed. A new field of investigation is also opening up on the effects of fathers' diets and their repercussions on the development of the offspring's phenotypes. In mammals, epigenetic changes in offspring have recently been shown to be dependent on the diet of male breeding stock (e.g., in mice). What about in birds?
The door is thus wide open for the future to program or redirect the metabolism of embryos at an early stage. Nevertheless, there are still current issues. Are these feeding strategies in line with societal expectations? Intervention on the diets of female reproducers may seem more applicable and ethically acceptable than an injection in the egg. However, when female reproducers are so restricted, what assurance do we have that the enriched elements in the diet will not be diverted for their own benefit? Let us not omit a third possibility to modulate and redirect the metabolism of animals early, nutrition and management of animals during the first week of life, a period in which animals still show some metabolic plasticity. Regardless of the approach used, maternal nutrition, in ovo nutrition or early nutrition, it is essential not to restrict oneself to profitability criteria that always favour heavier animals obtained more rapidly. In addition to early nutrition, other strategies around egg incubation (influence of temperature, light intensity, etc.) are possible and these may influence the metabolism of embryos and the subsequent performance of the animals.
Acknowledgments
This article was translated with www.DeepL.com/Translator and the authors used Proof-Reading Service (http://www.proof-reading-service.com) for English language editing.
References
- Adamstone F.B., 1931. The effects of vitamin E deficiency on the development of the chick. J. morphol., 52, 47-80.
- Adamstone F.B., 1941. Erythrophagocytosis in chicks reared on a vitamin E-deficient ration supplemented with halibut liver oil. Arch. Pathol., 31, 622-626.
- Adrian J., 1958. Répercussions des avitaminoses sur le développement embryonnaire chez le mammifère et l’oiseau. Ann. Zootech., INRA/EDP Sciences, 7, 97-122.
- Aigueperse N., Calandreau L., Bertin A., 2013. Maternal diet influences offspring feeding behavior and fearfulness in the precocial chicken. PLoS One, 8, e77583.
- Al-Murrani W.K., 1982. Effect of injecting amino acids into the egg on embryonic and subsequent growth in the domestic fowl. Br. Poult. Sci., 23, 171-174.
- Altmann S., Murani E., Schwerin M., Metges C.C., Wimmers K., Ponsuksili S., 2012. Maternal dietary protein restriction and excess affects offspring gene expression and methylation of non-SMC subunits of condensin I in liver and skeletal muscle. Epigenetics, 7, 239-252.
- Altmann S., Murani E., Schwerin M., Metges C.C., Wimmers K., Ponsuksili S., 2013. Dietary protein restriction and excess of pregnant German Landrace sows induce changes in hepatic gene expression and promoter methylation of key metabolic genes in the offspring. J. Nutr. Biochem., 24, 484-495.
- Anderson O.S., Sant K.E., Dolinoy D.C., 2012. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem., 23, 853-859.
- Araújo I.C.S., Café M.B., Noleto R.A., Martins J.M.S., Ulhoa C.J., Guareshi G.C., Reis M.M., Leandro N.S.M., 2018. Effect of vitamin E in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult Sci., 0, 1-10.
- Arnould C., Michel V., Le Bihan-Duval E., 2011. Sélection génétique et bien-être des poulets de chair et des reproducteurs. In : Bien-être du poulet de chair. Bouvarel I. (Ed). Dossier, INRA Prod. Anim., 24, 165-170.
- Baéza E., Chartrin P., Bordeau T., Lessire M., Thoby J.M., Gigaud V., Blanchet M., Alinier A., Leterrier C., 2017. Omega-3 polyunsaturated fatty acids provided during embryonic development improve the growth performance and welfare of Muscovy ducks (Cairina moschata). Poult. Sci., 96, 3176-3187.
- Bakyaraj S., Bhanja S.K., Majumdar S., Dash B., 2012. Modulation of post-hatch growth and immunity through in ovo supplemented nutrients in broiler chickens. J. Sci. Food Agric., 92, 313-320.
- Bello A., Zhai W., Gerard P.D., Peebles E.D., 2013. Effects of the commercial in ovo injection of 25-hydroxycholecalciferol on the hatchability and hatching chick quality of broilers. Poult. Sci., 92, 2551-2559.
- Bello A., Bricka R.M., Gerard P.D., Peebles E.D., 2014. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on broiler bone development and mineralization on days 0 and 21 posthatch. Poult. Sci., 93, 1053-1058.
- Bergoug H., Guinebretière M., Tong Q., Roulston N., Romanini C.E., Exadaktylos V., Berckmans D., Garain P., Demmers T.G., McGonnell I.M., Bahr C., Burel C., Eterradossi N., Michel V., 2013. Effect of transportation duration of 1-day-old chicks on postplacement production performances and pododermatitis of broilers up to slaughter age. Poult. Sci., 92, 3300-3309.
- Berri C., Besnard J., Relandeau C., 2008. Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult. Sci., 87, 480-484.
- Bertin A., Calandreau L., Arnould C., Lévy F., 2012. The developmental stage of chicken embryos modulates the impact of in ovo olfactory stimulation on food preferences. Chem. Senses, 37, 253-261.
- Bigot K., Mignon-Grasteau S., Picard M., Tesseraud S., 2003. Effects of delayed feed intake on body, intestine, and muscle development in neonate broilers. Poult. Sci., 82, 781-788.
- Bourin M.C., 2011. In : Caractérisation fonctionnelle et biochimique des protéases et antiprotéases présentes dans le jaune d’œuf Gallus gallus. Thèse soutenue en décembre 2011 à l’Université François Rabelais de Tours, France.
- Bourin M., Gautron J., Berges M., Attucci S., Le Blay G., Labas V., Nys Y., Rehault-Godbert S., 2011. Antimicrobial potential of egg yolk ovoinhibitor, a multidomain Kazal-like inhibitor of chicken egg. J. Agric. Food Chem., 59, 12368-12374.
- Breedlove C., Minc J.K., Tang D.C., van Santen V.L., van Ginkel F.W., Toro H., 2011. Avian influenza adenovirus-vectored in ovo vaccination: target embryo tissues and combination with Marek's disease vaccine. Avian Dis., 55, 667-673.
- Bruggeman V., Onagbesan O., D'Hondt E., Buys N., Safi M., Vanmontfort D., Berghman L., Vandesande F., Decuypere E., 1999. Effects of timing and duration of feed restriction during rearing on reproductive characteristics in broiler breeder females. Poult. Sci., 78, 1424-1434.
- Brun J.M., Basso B., Bernadet M.D., Cornuez A., Leroux S., Lessire M., Sellier N., Pitel F., Morisson M., 2013. Influence du taux de méthionine dans l'aliment de la cane commune sur les performances de gavage de ses descendants mulards : une question de programmation métabolique précoce et d’épigénétique ? In : Journ. Rech. Avicole et Palmipèdes à Foie Gras, 10, 215.
- Brun J.M., Bernadet M.D., Cornuez A., Leroux S., Bodin L., Basso B., Davail S., Jaglin M., Lessire M., Martin X., Sellier N., Morisson M., Pitel F., 2015. Influence of grand-mother diet on offspring performances through the male line in Muscovy duck. BMC Genet., 16, 145.
- Calini F., Sirri F., 2007. Breeder Nutrition and Offspring Performance. Brazilian J. Poult. Sci., 9, 77-83.
- Castanon J.I.R., 2007. History of the use of antibiotic as growth promoters in European Poultry Feeds. Poult. Sci., 11, 2466-2471.
- Cherian G., 2015. Nutrition and metabolism in poultry: role of lipids in early diet. J. Anim. Sci. Biotechnol., 6, 28.
- Chinrasri O., Chantiratikul P., Maneetong S., Chookhampaeng S., Chantiratikul A., 2013. Productivity and selenium concentrations in egg and tissue of laying quails fed selenium from hydroponically produced selenium-enriched kale sprout (Brassica oleracea var. alboglabra L.). Biol. Trace Elem. Res., 155, 381-386.
- Ciacciariello M., Tyler N.C., 2013. The effects of maternal dietary lysine intake on offspring performance to 21 days of age. J. Appl. Poult. Res., 22, 238-244.
- Coşkun I., Erener G., Şahin A., Ufuk Karadavut U., Altop A., Okur A.A., 2014. Impacts of In Ovo Feeding of DL-Methionine on Hatchability and Chick Weight. Turkish J. Agric. - Food Sci. Technol., 2, 47-50.
- DaSilva M., 2017. Les liquides amniotique et allantoïque de l'œuf de poule : caractérisation et rôle dans la protection de l'embryon au cours de l'incubation ». Thèse de l’université de Tours, soutenue en décembre 2017.
- Decuypere E., Bruggeman V., Barbato G.F., Buyse J., 2003. Growth an reproduction problems associated with selection for increased broiler meat production. In: Poultry Genetics, Breeding and Biotechnology. Muir W., Aggrey S.E. (Ed). Cabi Publishing, Wallingford, U.K., 13-28. http://base.dnsgb.com.ua/files/book/Agriculture/Animal-Agriculture/Poultry-Genetics-Breeding-and-Biotechnology.pdf
- Decuypere E., Hocking P.M., Tona K., Onagbesan O., Bruggeman V., Jones E.K.M., Cassy S., Rideau N., Metayer S., Jego Y., Putterflam J., Tesseraud S., Collin A., Duclos M., Trevidy J.J., Williams J., 2006. Broiler breeder paradox: a project report. World's Poult. Sci. J., 62, 443-453.
- Decuypere E., Bruggeman V., Everaert N., Li Y., Boonen R., De Tavernier J., Janssens S., Buys N., 2010. The broiler breeder paradox: ethical, genetic and physiological perspectives, and suggestions for solutions. Br. Poult. Sci., 51, 569-579.
- De Jong I.C., Guemene D., 2011. Major welfare issues in broiler breeders. World's Poult. Sci. Journal, 67, 73-81.
- Ding S.T., Lilburn M.S., 1996. Characterization of changes in yolk sac and liver lipids during embryonic and early posthatch development of turkey poults. Poult. Sci., 75, 478-483.
- Donohoe D.R., Bultman S.J., 2012. Metaboloepigenetics: interrelationships between energy metabolism and epigenetic control of gene expression. J. Cell. Physiol., 227, 3169-3177.
- Druyan S., 2010. The effects of genetic line (broilers vs. layers) on embryo development. Poult Sci., 89, 1457-1467.
- Ebrahimi M.R., Jafari Ahangari Y., Zamiri M.J., Akhlaghi A., Atashi H., 2012. Does preincubational in ovo injection of buffers or antioxidants improve the quality and hatchability in long-term stored eggs? Poult. Sci., 91, 2970-2976.
- Emmerson D.A., 1997. Commercial approaches to genetic selection for growth and feed conversion in domestic poultry. Poult. Sci., 76, 1121-1125.
- Everaert N., Métayer-Coustard S., Willemsen H., Han H., Song Z., Ansari Z., Decuypere E., Buyse J., Tesseraud S., 2013. The effect of albumen removal before incubation (embryonic protein under-nutrition) on the post-hatch performance, regulators of protein translation activation and proteolysis in neonatal broilers. Br. J. Nutr., 110, 265-274.
- Fagerberg B., Bondjers L., Nilsson P., 2004. Low birth weight in combination with catch-up growth predicts the occurrence of the metabolic syndrome in men at late middle age: the Atherosclerosis and Insulin Resistance study. J. Intern. Med., 256, 254-259.
- Favero A., Vieira S.L., Angel C.R., Bos-Mikich A., Lothhammer N., Taschetto D., Cruz R.F., Ward T.L., 2013. Development of bone in chick embryos from Cobb 500 breeder hens fed diets supplemented with zinc, manganese, and copper from inorganic and amino acid-complexed sources. Poult. Sci., 92, 402-411.
- Ferguson T.M., Couch J.R., 1954. Further gross observations on the B12-deficient chick embryo. J. Nutr., 54, 361-370.
- Foye O.T., Ferket P.R., Uni Z., 2007. The effects of in ovo feeding arginine, beta-hydroxy-beta-methyl-butyrate, and protein on jejunal digestive and absorptive activity in embryonic and neonatal turkey poults. Poult. Sci., 86, 2343-2349.
- Frésard L., Morisson M., Brun J.M., Collin A., Pain B., Minvielle F., Pitel F., 2013. Epigenetics and phenotypic variability: some interesting insights from birds. Genet. Sel. Evol., 45, 16.
- Gao T., Zhao M., Zhang L., Li J., Yu L., Lv P., Gao F., Zhou G., 2017. Effect of in ovo feeding of l-arginine on the hatchability, growth performance, gastrointestinal hormones, and jejunal digestive and absorptive capacity of posthatch broilers. J. Anim. Sci., 95, 3079-3092.
- Ghanaatparast-Rashti M., Mottaghitalab M., Ahmadi H., 2018. In ovo feeding of nutrients and its impact on post-hatching water and feed deprivation up to 48 hr, energy status and jejunal morphology of chicks using response surface models. J. Anim. Physiol. Anim. Nutr. (Berl), 102, e806-e817.
- Goel A., Bhanja S.K., Pande V., Mehra M., Mandal A., 2013. Effects of in ovo administration of vitamins on post hatch-growth, immunocompetence and blood biochemical profiles of broiler chickens. Indian J. Anim. Sci., 83, 916-921.
- Gonzalez-Esquerra R., Leeson S., 2000. Studies on the metabolizable energy content of ground full-fat flaxseed fed in mash, pellet, and crumbled diets assayed with birds of different ages. Poult. Sci., 79, 1603-1607.
- Gore A.B., Qureshi M.A., 1997. Enhancement of humoral and cellular immunity by vitamin E after embryonic exposure. Poult. Sci., 76, 984-991.
- Grodzik M., Sawosz F., Sawosz E., Hotowy A., Wierzbicki M., Kutwin M., Jaworski S., Chwalibog A., 2013. Nano-nutrition of chicken embryos. The effect of in ovo administration of diamond nanoparticles and L-glutamine on molecular responses in chicken embryo pectoral muscles.Int. J. Mol. Sci., 14, 23033-23044.
- Guernec A., Berri C., Chevalier B., Wacrenier-Cere N., Le Bihan-Duval E., Duclos M.J., 2003. Muscle development, insulin-like growth factor-I and myostatin mRNA levels in chickens selected for increased breast muscle yield. Growth Horm. IGF Res., 13, 8-18.
- Havenstein G.B., Ferket P.R., Qureshi M.A., 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci., 82, 1500-1508.
- Henry M.H., Burke W.H., 1999. The effects of in ovo administration of testosterone or an antiandrogen on growth of chick embryos and embryonic muscle characteristics. Poult. Sci., 78, 1006-1013.
- Ho D.H., Reed W.L., Burggren W.W., 2011. Egg yolk environment differentially influences physiological and morphological development of broiler and layer chicken embryos. J. Exp. Biol., 214, 619-628.
- Hocking P.M., 2010. Developments in poultry genetic research 1960-2009. Br. Poult. Sci., 51 Suppl 1, 44-51.
- Jiakui L., Xiaolong W., 2004. Effect of dietary organic versus inorganic selenium in laying hens on the productivity, selenium distribution in egg and selenium content in blood, liver and kidney. J. Trace Elem. Med. Biol., 18, 65-68.
- Kadam M.M., Bhanja S.K., Mandal A.B., Thakur R., Vasan P., Bhattacharyya A., Tyagi J.S., 2008. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens. Br. Poult. Sci., 49, 736-741.
- Kadam M.M., Barekatain M.R., Bhanja S.K., Iji P.A., 2013. Prospects of in ovo feeding and nutrient supplementation for poultry: the science and commercial applications--a review. J. Sci. Food Agric., 93, 3654-3661.
- Kidd M.T., 2003. A treatise on chicken dam nutrition that impacts on progeny. World Poult. Sci. J., 59, 475-494.
- Kidd M.T., 2004. Nutritional modulation of immune function in broilers. Poult Sci., 83, 650-657.
- Kidd M.T., McDaniel C.D., Peebles E.D., Barber S.J., Corzo A., Branton S.L., Woodworth J.C., 2005. Breeder hen dietary L-carnitine affects progeny carcase traits. Br. Poult. Sci., 46, 97-103.
- Kocamis H., Yeni Y.N., Kirkpatrick-Keller D.C., Killefer J., 1999. Postnatal growth of broilers in response to in ovo administration of chicken growth hormone. Poult. Sci., 78, 1219-1226.
- Kocamis H., Yeni Y.N., Brown C.U., Kenney P.B., Kirkpatrick-Keller D.C., Killefer J., 2000. Effect of in ovo administration of insulin-like growth factor-I on composition and mechanical properties of chicken bone. Poult. Sci., 79, 1345-1350.
- Koppenol A., Buyse J., Everaert N., Willems E., Wang Y., Franssens L., Delezie E., 2015. Transition of maternal dietary n-3 fatty acids from the yolk to the liver of broiler breeder progeny via the residual yolk sac. Poult. Sci. 94, 43-52.
- Kornasio R., Halevy O., Kedar O., Uni Z., 2011. Effect of in ovo feeding and its interaction with timing of first feed on glycogen reserves, muscle growth, and body weight. Poult. Sci., 90, 1467-1477.
- Kucharska-Gaca J., Kowalska E., Dębowska M., 2017. In ovo feeding – technology of the future – a review. Annals Anim. Sci., doi:10.1515/aoas-2017-0004
- Lee S.H., Lillehoj H.S., Jang S.I., Jeong M.S., Xu S.Z., Kim J.B., Park H.J., Kim H.R., Lillehoj E.P., Bravo D.M., 2014. Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens. Poult. Sci., 93, 1113-1121.
- Leeson S., Caston L., MacLaurin T., 1998. Organoleptic evaluation of eggs produced by laying hens fed diets containing graded levels of flaxseed and vitamin E. Poult. Sci., 77, 1436-1440.
- Lesuisse J., Li C., Schallier S., Leblois J., Everaert N., Buyse J., 2017. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult Sci., 96, 3949-3959.
- Lesuisse J., Li C., Schallier S., Clímaco W.L.S., Bautil A., Everaert N., Buyse J., 2018. Multigenerational effects of a reduced balanced protein diet during the rearing and laying period of broiler breeders. 1. Performance of the F1 breeder generation. Poult Sci., 97, 1651-1665.
- Lesuisse J., Schallier S., Li C., Bautil A., Li B., Leblois J., Buyse J., Everaert N., 2018. Multigenerational effects of a reduced balanced protein diet during the rearing and laying period of broiler breeders. 2. Zootechnical performance of the F1 broiler offspring. Poult Sci., 97, 1666-1676.
- Li Y., Wang Y., Willems E., Willemsen H., Franssens L., Buyse J., Decuypere E., Everaert N., 2016. In ovo L-arginine supplementation stimulates myoblast differentiation but negatively affects muscle development of broiler chicken after hatching. J. Anim. Physiol. Anim. Nutr. (Berl), 100, 167-177.
- Li C., Lesuisse J., Schallier S., Clímaco W., Wang Y., Bautil A., Everaert N., Buyse J., 2018. The effects of a reduced balanced protein diet on litter moisture, pododermatitis and feather condition of female broiler breeders over three generations. Animal, 12, 1493-1500.
- Maloney C.A., Hay S.M., Reid M.D., Duncan G., Nicol F., Sinclair K.D., Rees W.D., 2013. A methyl-deficient diet fed to rats during the pre- and peri-conception periods of development modifies the hepatic proteome in the adult offspring. Genes Nutr., 8, 181-190.
- McGruder B.M., Zhai W., Keralapurath M.M., Bennett L.W., Gerard P.D., Peebles E.D., 2011. Effects of in ovo injection of electrolyte solutions on the pre- and posthatch physiological characteristics of broilers. Poult. Sci., 90, 1058-1066.
- Menge H., Littlefield L.H., Frobish L.T., Weinland B.T., 1974. Effect of cellulose and cholesterol on blood and yolk lipids and reproductive efficiency of the hen. J. Nutr., 104, 1554-1566.
- Métayer S., Seiliez I., Collin A., Duchêne S., Mercier Y., Geraert P.A., Tesseraud S., 2008. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J. Nutr. Biochem., 19, 207-215.
- Métayer-Coustard S., Mameri H., Seiliez I., Crochet S., Crépieux P., Mercier Y., Geraert P.A., Tesseraud S., 2010. Methionine deprivation regulates the S6K1 pathway and protein synthesis in avian QM7 myoblasts without activating the GCN2/eIF2 alpha cascade. J. Nutr., 140, 1539-1545.
- Métayer-Coustard S., Lesuisse J., Schallier S., Roffidal L., Buyse J., 2017. Alimentation maternelle et nutrition in ovo, de nouvelles stratégies alimentaires précoces au service de la production avicole. Journ. Rech. Avicole et Palmipèdes à Foie Gras, 12, 67-77.
- Moran E.T. Jr., 2007. Nutrition of the developing embryo and hatchling. Poult. Sci., 86, 1043-1049.
- Moss F.P., 1968. The relationship between the dimension of the fibres and the number of nuclei during normalgrowth of skeletal muscle in the domestic fowl. Am. J. Anat., 122, 555-564.
- Müller Fernandes J.I., Murakami A.E., Gomes de Souza L.M., Ospina-Rojas I.C., Rossi R.M.., 2014. Effect of arginine supplementation of broiler breeder hens on progeny performance. Can. J. Anim. Sci., 94, 313-321.
- Neves D.G., Retes P.L., Rocha R.R., Ferreira L.G., Naves L.P., Alvarenga R.R., Fassani E.J., Pereira L.J., Sousa R.V., Zangeronimo M.G., 2017. Effects of in ovo feeding with glycerol for broilers. J. Anim. Physiol. Anim. Nutr., 101, 434-440.
- Noble R.C., Connor K., Smith W.K., 1984. The synthesis and accumulation of cholesteryl esters by the developing embryo of the domestic fowl. Poult. Sci., 63, 558-564.
- Ohta Y., Tsushima N., Koide K., Kidd M.T., Ishibashi T., 1999. Effect of amino acid injection in broiler breeder eggs on embryonic growth and hatchability of chicks. Poult. Sci., 78, 1493-1498.
- Ohta Y., Kidd M.T., Ishibashi T., 2001. Embryo growth and amino acid concentration profiles of broiler breeder eggs, embryos, and chicks after in ovo administration of amino acids. Poult. Sci., 80, 1430-1436.
- Pearce J., 1971. Carbohydrate metabolism in the domestic fowl. Proc Nutr Soc., 30, 254-259. Review.
- Peebles E.D., 2018. In ovo applications in poultry: A review. Poult. Sci., 97, 2322-2338.
- Polk H.D., Sipe G.R., 1940. The Effect of Vitamin A Deficiency on Malposition of the Chick Embryo. Poult. Sci., 19, 396-400.
- Powrie W.D., Nakaï S., 1986. The chemistry of eggs and egg products. In: Stadelman W.J., Cotterill O.J. (Eds). Egg science and technology. Avi Publishing, Westport, CT, 97-139.
- Pulikanti R., Peebles E.D., Keirs R.W., Bennett L.W., Keralapurath M.M., Gerard P.D., 2010. Pipping muscle and liver metabolic profile changes and relationships in broiler embryos on days 15 and 19 of incubation. Poult. Sci., 89, 860-865.
- Rao K., Xie J., Yang X., Chen L., Grossmann R., Zhao R., 2009. Maternal low-protein diet programmes offspring growth in association with alterations in yolk leptin deposition and gene expression in yolk-sac membrane, hypothalamus and muscle of developing Langshan chicken embryos. Br. J. Nutr., 102, 848-857.
- Retes P.L., Clemente A.H.S., Neves D.G., Espósito M., Makiyama L., Alvarenga R.R., Pereira L.J., Zangeronimo M.G., 2018. In ovo feeding of carbohydrates for broilers-a systematic review. J. Anim. Physiol. Anim. (Berl).102, 361-369. Review.
- Roto S.M., Kwon Y.M., Ricke S.C., 2016. Applications of In Ovo Technique for the Optimal Development of the Gastrointestinal Tract and the Potential Influence on the Establishment of Its Microbiome in Poultry. Front. Vet. Sci., 3, 63. Review.
- Rühl R., 2007. Effects of dietary retinoids and carotenoids on immune development. Proc. Nutr. Soc., 66, 458-469.
- Matin H.R.H., 2014. In ovo injection of vitamin E on post-hatch immunological parameters and broiler chicken performance. Asian Pacific J. Tropical Biomed., 4, S616-S619.
- Saunders-Blades J.L., Korver D.R., 2015. Effect of hen age and maternal vitamin D source on performance, hatchability, bone mineral density, and progeny in vitro early innate immune function. Poult. Sci., 94, 1233-1246.
- Scanes C.G., Griminger P., 1990. Endocrine-nutrition interactions in birds. J. Exp. Zool. Suppl., 4, 98-105.
- Shafey T.M., Mahmoud A.H., Alsobayel A.A., Abouheif M.A., 2014. Effects of “in ovo” administration of amino acids on hatchability and performance of meat chickens. S. Afr. J. Anim. Sci., 44.
- Simmons R., 2011. Epigenetics and maternal nutrition: nature v. nurture. Proc. Nutr. Soc., 70, 73-81.
- Sinclair K.D., Allegrucci C., Singh R., Gardner D.S., Sebastian S., Bispham J., Thurston A., Huntley J.F., Rees W.D., Maloney C.A., Lea R.G., Craigon J., McEvoy T.G., Young L.E., 2007. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. U S A., 104, 19351-19356.
- Smirnov A., Tako E., Ferket P.R., Uni Z., 2006. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci., 85, 669-673.
- Soares J.H. Jr, Kerr J.M., Gray R.W., 1995. 25-hydroxycholecalciferol in poultry nutrition. Poult. Sci., 74, 1919-1934.
- Spratt R.S., Leeson S., 1987. Effect of protein and energy intake of broiler breeder hens on performance of broiler chicken offspring. Poult. Sci., 66, 1489-1494.
- Sunny N.E., Bequette B.J., 2011. Glycerol is a major substrate for glucose, glycogen, and nonessential amino acid synthesis in late-term chicken embryos. J. Anim. Sci., 89, 3945-3953.
- Surai P.F., Fisinin V.I., Karadas F., 2016. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr., 2, 1-11.
- Surai P.F., Sparks N.H., 2001. Comparative evaluation of the effect of two maternal diets on fatty acids, vitamin E and carotenoids in the chick embryo. Br. Poult. Sci., 42, 252-259.
- Tako E., Ferket P.R., Uni Z., 2004. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult. Sci., 83, 2023-2028.
- Tesseraud S., Bigot K., Taouis M., 2003. Amino acid availability regulates S6K1 and protein synthesis in avian insulin-insensitive QM7 myoblasts. FEBS Lett., 540, 176-180.
- Tesseraud S., Métayer Coustard S., Collin A., Seiliez I., 2009. Role of sulfur amino acids in controlling nutrient metabolism and cell functions: implications for nutrition. Br. J. Nutr., 101, 1132-1139.
- Tesseraud S., Chartrin P., Métayer-Coustard S., Hermier D., Simon N., Peyronnet C., Lessire M., Baéza E., 2014. Modulation of the insulin anabolic signalling cascade in growing chickens by n-3 PUFA. Br. J. Nutr., 111, 761-772.
- Tona K., Bamelis F., De Ketelaere B., Bruggeman V., Moraes V.M., Buyse J., Onagbesan O., Decuypere E., 2003. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci., 82, 736-741.
- Tong B.C., Barbul A., 2004. Cellular and physiological effects of arginine. Mini Rev Med Chem., 4, 823-832.
- Torres CA, Korver DR., 2018. Influences of trace mineral nutrition and maternal flock age on broiler embryo bone development. Poult. Sci., 97, 2996-3003.
- Uni Z., Ferket P.R., Tako E., Kedar O., 2005. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci., 84, 764-770.
- van der Waaij E.H., van den Brand H., van Arendonk J.A., Kemp B., 2011. Effect of match or mismatch of maternal-offspring nutritional environment on the development of offspring in broiler chickens. Animal, 5, 741-748.
- Velleman S.G., 2007. Muscle development in the embryo and hatchling. Poult. Sci., 86, 1050-1054.
- Veron V., Marandel L., Liu J., Vélez E.J., Lepais O., Panserat S., Skiba S., Seiliez I., 2018. DNA methylation of the promoter region of bnip3 and bnip3l genes induced by metabolic programming. BMC Genomics., 19, 677.
- Wang Y.W., Sunwoo H., Cherian G., Sim J.S., 2004. Maternal dietary ratio of linoleic acid to alpha-linolenic acid affects the passive immunity of hatching chicks. Poult. Sci., 83, 2039-2043.
- Yair R., Uni Z., 2011. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult Sci., 90, 1523-1531.
- Yair R., Shahar R., Uni Z., 2013. Prenatal nutritional manipulation by in ovo enrichment influences bone structure, composition, and mechanical properties. J. AnimSci., 91, 2784-2793.
- Yair R., Shahar R., Uni Z., 2015. In ovo feeding with minerals and vitamin D3 improves bone properties in hatchlings and mature broilers. Poult Sci., 94, 2695-2707.
- Yassin H., Velthuis A.G., Boerjan M., van Riel J., 2009. Field study on broilers' first-week mortality. Poult. Sci., 88, 798-804.
- Yu L.L., Gao T., Zhao M.M., Lv P.A., Zhang L;, Li J.L., Jiang Y., Gao F., Zhou G.H., 2018. In ovo feeding of L-arginine alters energy metabolism in post-hatch broilers. Poult Sci., 97, 140-148.
- Zhao M.M., Gao T., Zhang L., Li J.L., Lv P.A., Yu L.L., Gao F., Zhou G.H., 2017. Effects of in ovo feeding of creatine pyruvate on the hatchability, growth performance and energy status in embryos and broiler chickens. Animal, 11, 1689-1697.
- Zhai W., Bennett L.W., Gerard P.D., Pulikanti R., Peebles E.D., 2011c. Effects of in ovo injection of carbohydrates on somatic characteristics and liver nutrient profiles of broiler embryos and hatchlings. Poult. Sci., 90, 2681-2688.
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E., 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci., 93, 2970-2982.
Abstract
The development of the bird embryo depends on the environment in which it is immersed and the nutrients available. The first post-hatching week is often tricky, with sometimes significant postnatal mortalities due to feed delays and suboptimal environmental and transport conditions. Links between parental nutrition, egg composition and subsequent animal behaviour, performance, and disease susceptibility are well established. Nutrient supplementations into the egg via maternal feeding or in ovo- injections ("in ovo feeding") are thus innovative strategies to optimize nutrient supply to the embryo. The aim of such approaches is to optimize the nutritional supply of breeders or embryos in order to obtain better chick quality in terms of robustness, growth and / or body composition by taking advantage of the embryonic plasticity for nutrient utilization. The precocious manipulation of the environment in which the embryo develops is very promising for analyzing the influence of early conditions on the development of long-term phenotypes whether it is on criteria of growth, body composition, tissue characteristics, and robustness towards challenges or adaptation to different breeding conditions. In addition to the benefits observed for animals, these dietary strategies also attempt to respond to environmental and economic interests.
Attachments
No supporting information for this article##plugins.generic.statArticle.title##
 Views: 29021
Views: 29021
Downloads
 PDF: 1779
PDF: 1779
Most read articles by the same author(s)
- Sophie REHAULT-GODBERT, Marie BOURIN, Joël GAUTRON, Maxime QUENTIN, End of culling day-old male layer chicks: ovosexing as the method of choice , INRAE Productions Animales: Vol. 36 No. 4 (2023)
- Anne COLLIN, Vincent COUSTHAM, Jacob Kokou TONA, Sophie TESSERAUD, Sandrine MIGNON-GRASTEAU, Bertrand MÉDA, Anaïs VITORINO CARVALHO, Yann GUYOT, Sandrine LAGARRIGUE, Frédérique PITEL, Tatiana ZERJAL, What mitigation and adaptation strategies for poultry production in the face of climate change? , INRAE Productions Animales: Vol. 37 No. 1 (2024)
- Vincent COUSTHAM, Charlotte, Chloé, Anne, Ingrid, Julie DEMARS, Guillaume DEVAILLY, Mireille MORISSON, Marianne HOUSSIER, Sandrine LAGARRIGUE, Sonia MÉTAYER-COUSTARD, Sandrine MIGNON-GRASTEAU, Stéphane PANSERAT, Angélique PETIT, Anaïs VITORINO CARVALHO, Tatiana ZERJAL, Frédérique PITEL, Epigenetics, genes and the environment: what importance for breeding practices and selection methods in poultry? , INRAE Productions Animales: Vol. 36 No. 4 (2023)
- Sophie TESSERAUD, Isabelle BOUVAREL, P. FRAYSSE, Sophie MÉTAYER COUSTARD, A. COLLIN, M. LESSIRE, C. BERRI, Adapting protein-energy metabolism to optimize poultry carcass and meat quality , INRAE Productions Animales: Vol. 27 No. 5 (2014)

