Introduction
Faced with the development of antimicrobial resistance, major efforts have been made over the past ten years in France to optimise the use of antimicrobials in the livestock sector. A previous article (David et al., 2019) discussed the situation in the French cattle industry. In monogastric livestock (pigs, poultry and rabbits), indicators used to monitor veterinarian drug sales show that animal exposure to antimicrobials increased up to the mid-2000s (Anses, 2021a). In response to this rise, health and animal stakeholders mobilised, undertaking a series of coordinated actions within the framework of the first EcoAntibio plan (2012-2017).
Monogastric species are mainly reared in standardised intensive production systems, in which the use of antimicrobials once was an effective tool for controlling common livestock diseases, especially during delicate phases (e.g. chick settling or piglet weaning). In the past, antimicrobials were used regularly in a metaphylactic approach (treatment of the entire batch of animals when a few individuals are ill), or even prophylactically (treatment as a preventive measure, during a risk period, for diseases occurring at a specific age and on a recurring basis on certain farms). Changing this vision of antimicrobial use would have to address certain technical constraints (ageing buildings), economic constraints (cost-benefit ratio of antimicrobial therapy and alternative approaches), and the weight of habits.
Changing practices requires the removal of obstacles and the evolution, in parallel with the technical progress achieved, of the perceptions of farmers and their technical-sanitary supervisors regarding animal health, their understanding of antimicrobial therapy and its impact, and their apprehensions about eliminating antimicrobial use, which is considered as a kind of 'safety insurance'. Nevertheless, the structure of these organised sectors means that incentives and actions undertaken at the level of the entire sector or of production organisations can have an effective and rapid impact.
The aim of this article is to review the use and evolution of antimicrobials in the monogastric sector in France, to present the efforts undertaken and the different approaches developed since the 2000s in terms of the prevention of health problems and evolution of antimicrobial therapy practices, and to consider the prospects for the future.
1. Evolution of antimicrobial use in the monogastric sector
1.1 National monitoring of antimicrobial sales
The tonnage of antimicrobials sold (Anses, 2021a) has decreased considerably since 1999, when the monitoring of antimicrobial sales based on manufacturers' declarations first began. However, these tonnages do not accurately reflect antimicrobial use levels. To monitor usage accurately, it is necessary to consider both the animals’ exposure to antimicrobials, taking into account the dosage and duration of administration of the different antimicrobials, and the evolution of the animal population over time. The ALEA (Animal Level of Exposure to Antimicrobials) indicator, which relates the live weight of treated animals to that of all animals - the potentially exposed population - is the most frequently used exposure indicator in France. Between 2011 and 2020, it decreased, all classes of antimicrobials combined, by 56% for pigs, 64% for poultry and 40% for rabbits. Pigs and poultry are mainly treated orally. The use of premixed drugs is decreasing and the ALEA of this pharmaceutical form had decreased by 78%, 69% and 55% in 2020 compared to 2011 for pigs, poultry and rabbits, respectively (Anses, 2021a).
The classes of antimicrobials used and the associated changes differ between species. According to 2020 ALEA values, pigs were treated mainly with tetracyclines, penicillins, polymyxins, followed by macrolides, sulphonamides and trimethoprim. Poultry were treated mainly with polymyxins, penicillins and tetracyclines, then sulphonamides and trimethoprim. Rabbits were treated mainly with tetracyclines, sulphonamides and trimethoprim, followed by aminoglycosides, polypeptides and pleuromutilines.
Exposure to critically important antimicrobials – meaning ones used as a last resort in the treatment of certain infectious diseases in humans - decreased dramatically compared to 2013 data. In 2020, for fluoroquinolones, the decrease was 92% in pigs and 76% in poultry, and for 3rd and 4th generation cephalosporins (not authorised for poultry), the decrease was 96% in pigs. Exposure to colistin, which is not listed as a critically important antimicrobial but is subject to enhanced monitoring, decreased by 75% for pigs and 63% for poultry compared to the average exposure calculated for 2014 and 2015. The objective set by the 2017-2021 EcoAntibio 2 plan to reduce exposure to this antimicrobial by 50% over five years therefore was exceeded in the pig and poultry sectors, which are the main users.
1.2 Measuring antimicrobial use
Different systems can be used to complete the overview obtained from monitoring antimicrobial sales (Anses, 2021a); these include studying data at a finer scale (farms) and adding further information (physiological stage, reason for use, etc.). In addition to monitoring sales, three types of systems can be identified in France (Table 1).
The panels and observatories run by interprofessional organisations and technical institutes, and initiated with the assistance of the French Agency for Food, Environmental and Occupational Health and Safety (ANSES), aim to provide reference data on antimicrobial use based on a sample of farms on which various exposure indicators are calculated using a standardized method. The INAPORC panel (Hémonic et al., 2019) measures antimicrobial use on a representative sample of randomly selected pig farms. The data, collected periodically (in 2010, 2013, 2016, 2019), make it possible to describe changes in antimicrobial use by molecule, pharmaceutical form, physiological stage, target disease and type of treatment (preventive, metaphylactic, curative). The RefA²vi network (Rousset et al., 2019) has similar objectives for the poultry industry (turkeys and broilers, all types of production combined). In 2018, a first pilot phase of data collection was carried out with 11 voluntary production organisations. In Label Rouge poultry production, a collection system initiated by SYNALAF (Syndicat National des Labels Avicoles de France) provides a quarterly indicator of the frequency of antimicrobial use, distinguishing between the different production phases and certain families of antimicrobials. Since 2010, the rabbit industry has been equipped with a tool to quantitatively monitor antimicrobial use through the recording of IFTAs (Index of Frequency of Antimicrobial Treatment) for each rabbit flock (Fortun-Lamothe et al., 2011) under a national plan initiated by CLIPP (the French Interprofessional Committee for Broiler Rabbits).
Specific studies and surveys, based on data collected on farms or from veterinarians, make it possible to obtain a detailed analysis of antimicrobial use and to study the effect of different determinants of this use. Under the MINAPIG project, the study of antimicrobial use on 227 pig farms in four European countries showed that many factors are associated with use, and that it is difficult to identify generic explanatory elements. One of the main determinants was the occurrence of clinical respiratory or nervous signs in growing pigs (post-weaning and fattening) (Collineau et al., 2018).
Software for monitoring use in livestock farming enables farmers and veterinarians to continuously monitor the use of veterinary medicines (antimicrobials, but also vaccines, deworming agents, etc.) in real time. There are many purely private initiatives to collect data, either from veterinarians based on their prescriptions (INDICAVET, COOPERL, EVELUP, Certiferme software for the Michel Group, for example) or from farmers based on the record of the treatments they have administered. The GVET (Gestion des traitements vétérinaires) approach, developed for pig farming by IFIP, ANSES and ISAGRI, is a computerised version of the register of treatments administered on the farm (quantities of treatment administered, dates, reasons and animals concerned) which aims to replace the paper-based system.
Table 1. Devices for measuring antimicrobial use in livestock.
Forces |
Weaknesses |
|
|---|---|---|
ANSES-ANMV - |
Exhaustiveness of the data collected |
Low level of detail |
Panels and observatories |
Representativeness |
One-off view (quarterly, |
Studies, |
Representativeness |
Point-in-time view (cross-sectional |
Livestock monitoring software |
Continuous collection of data from veterinary prescriptions and/or from the register of treatments in the farm |
Heterogeneous calculation methods the indicators |
1.3. Variability of antimicrobial use in livestock
a. Poultry production: diversity of evolutionary paths between species and productions
The French poultry industry is characterised by a wide variety of species (broilers, turkeys, laying hens, palmipeds, guinea fowl, pigeons, quails, etc.) and production methods (operating or not under a quality label, providing or not access outdoors). One of the main limitations of the ANSES-ANMV sales monitoring data is the aggregation of data concerning all these species and production methods in the same 'poultry' category. In addition, data from the RefA²vi observatory show a difference in exposure between the two species mainly raised for meat (chicken and turkey) (RefA2vi, 2019).
In broiler turkey, a pharmacoepidemiological study (Vove, 2019) conducted on 1,209 batches between January 2015 and December 2017 reveals that, regardless of the type of production (standard, certified baby), all batches studied received at least one antimicrobial treatment. The most commonly used families of antimicrobials were the betalactams (amoxicillin, ampicillin and penicillin), cyclins and polypeptides (colistin). Exposure to colistin dropped considerably, with the percentage of batches treated falling from 79% to 39% in three years.
In standard broiler chicken, the RefA²vi observatory, considering data equivalent to 37% of broiler production, found a reduction in antimicrobial exposure of 30% to 32% between 2018 and 2020 depending on the indicators used (nDDkg
b. Pig production: marked differences between physiological stages
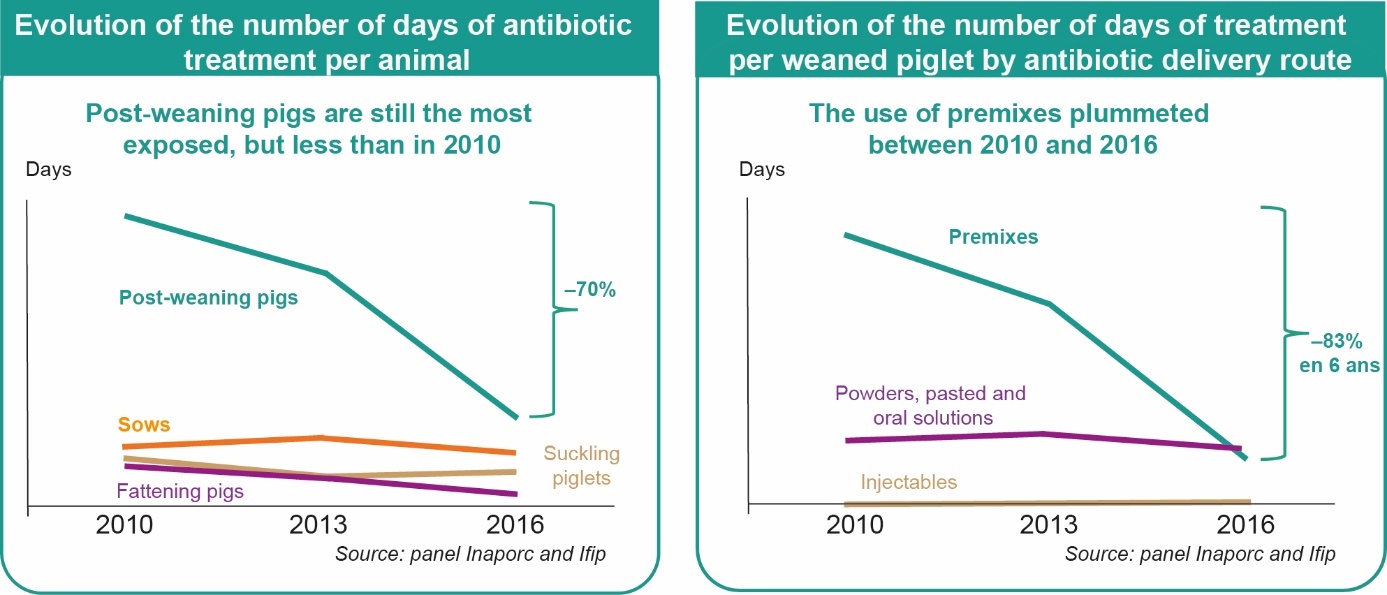
In pig production, it is widely recognised that antimicrobial use is not evenly distributed between the different production stages (Sjölund et al., 2016, Hémonic et al., 2019). Data from the INAPORC panel (Figure 1) show how the trajectories of usage change differ between physiological stages (Hémonic et al., 2019). In 2016, post-weaning piglets remained the physiological stage using the most antimicrobials (47% of total treatment days on the farm), mainly for digestive problems (52% of total piglet exposure time) ahead of respiratory problems (20% of total). However, the overall use of antimicrobials in this segment of production decreased by 70% between 2010 and 2016, with a more pronounced evolution between 2013 and 2016 (-63%) than between 2010 and 2013 (-19%). The most striking result is the sharp decline in the use of premixes, particularly colistin-based premixes. The rate of farms using premixes fell from 84% to 32% between 2010 and 2016, and the exposure time of piglets was reduced by 83%. This is partly due to the European Commission's decision in March 2015 to remove the indications for preventive use of oral colistin and to limit treatments to seven days (EMA, 2015). This reduction in colistin premixes has not resulted in a shift in use to other oral routes, nor to other digestive antimicrobials. Compensation with a zinc oxide premix, authorised in France in January 2016 and then banned again, was only slightly widespread (16% of farms concerned). This result suggests that digestive problems were managed by other preventive measures, such as vaccination, feeding, biosecurity or other aspects of farm management.
Overall antimicrobial use in fattening pigs also decreased by 71% between 2010 and 2016, with a more pronounced decrease between 2013 and 2016 than between 2010 and 2013 (Hémonic et al., 2019). This resulted in a decrease in the exposure times per animal and the percentage of farms affected by each type of treatment. Respiratory disorders were the most common reason for treatment.
Figure 1. Evolution of antimicrobial exposure in pigs (Source: INAPORC Panel, 143 farms).
In farrowing sows, which accounted for 29% of total antimicrobial exposure time on farms in 2016 (Hémonic et al., 2019), overall antimicrobial use decreased by 7% between 2010 and 2016. The major reason for use was urogenital disorders. Use also decreased in piglets in the maternity ward (28% decrease between 2010 and 2016). This decrease took place over the period 2010 - 2013, with use remaining stable between 2013 and 2016 (+ 1%). For sows and piglets in the maternity ward, the drop in the use of critically important antimicrobials (CIA) was very clear over six years (respectively -80% and -83% for fluoroquinolones and -100% and -98% for last generation cephalosporins). Two reasons are the moratorium established by veterinarians and breeders in 2010 for cephalosporins (Verliat et al., 2021), followed by the decree issued in 2016 extending the restriction of use to other CIAs such as fluoroquinolones (Decree n° 2016-317). For piglets in the maternity ward, the other major result is the cessation of the administration of antimicrobial premixes, which was a risky practice in terms of antimicrobial underdosing due to the small quantities of solid "first age" feed ingested by these animals.
c. Rabbit production: initial use differs little from one farm to another
Most rabbit farms in France are farrow-to-finish. A study carried out in 2009 and 2010 on 113 farms (Chauvin et al., 2011) found that female rabbits were more exposed to antimicrobials than male rabbits, with a mainly respiratory target for female rabbits and a mainly digestive target for male rabbits - the period around weaning being a critical stage for the latter. The same study revealed a high initial use, but also a clear decrease between the two years of the study: a 15% decrease in the amount of active substances was noted between 2009 and 2010 on 91 farms.
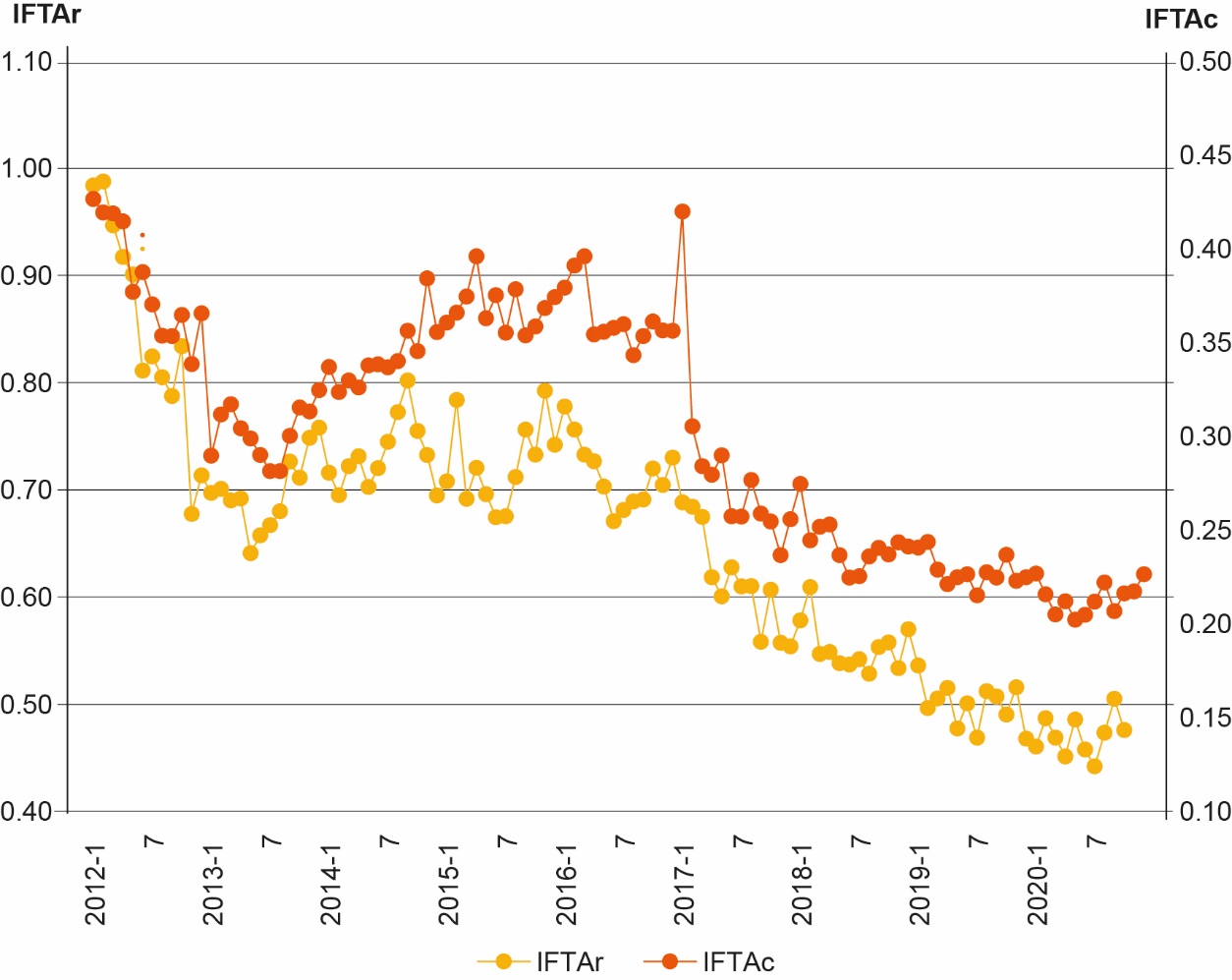
Since this study, sector professionals have been monitoring IFTA indicators to measure the use of antimicrobials on breeding animals (IFTAr) and on growing animals (IFTAc). These indicators are calculated from the treatments given to rabbits regardless of whether the products’ marketing authorisation is for rabbits or for other species. The curves (Figure 2) show a 47% drop in IFTAr and 44% drop in IFTAc between 2012 and 2020. A clear plateau is observed between 2014 and 2017, which can be linked to a very unfavourable sanitary context (epizootic of Viral Haemorrhagic Disease due to a new viral genotype); however, the drop resumes after 2017. It should be noted, however, that the exposure of rabbits to antimicrobials has remained stable for several years according to the ALEA indicator used by ANMV (Anses, 2021a) on a national scale, calculated on sales declared by the pharmaceutical laboratories.
Figure 2. Evolution of antimicrobial use in rabbits between 2012 and 2020 (IFTAr for breeding females and IFTAc for growing animals) (ITAVI-CLIPP data).